Anticancer Therapy-Related Increases in Arterial Stiffness: A Systematic Review and Meta-Analysis
- PMID: 32648507
- PMCID: PMC7660726
- DOI: 10.1161/JAHA.119.015598
Anticancer Therapy-Related Increases in Arterial Stiffness: A Systematic Review and Meta-Analysis
Abstract
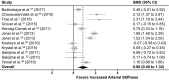
Background Cardio-oncology is a clinical discipline focused primarily on the early detection of anticancer therapy-related cardiomyopathy. However, there is growing evidence that the direct adverse consequences extend beyond the myocardium to affect the vasculature, but this evidence remains limited. In addition, there remains a paucity of clinically based strategies for monitoring vascular toxicity in these patients. Importantly, arterial stiffness is increasingly recognized as a surrogate end point for cardiovascular disease and may be an important vascular outcome to consider. Therefore, the aim of this systematic review and meta-analysis was to summarize evidence of increased arterial stiffening with anticancer therapy and evaluate the effect of treatment modifiers. Methods and Results A total of 19 longitudinal and cross-sectional studies that evaluated arterial stiffness both during and following anticancer therapy were identified using multiple databases. Two separate analyses were performed: baseline to follow-up (12 studies) and control versus patient groups (10 studies). Subgroup analysis evaluated whether stiffness differed as a function of treatment type and follow-up time. Standard mean differences and mean differences were calculated using random effect models. Significant increases in arterial stiffness were identified from baseline to follow-up (standard mean difference, 0.890; 95% CI, 0.448-1.332; P<0.0001; mean difference, 1.505; 95% CI, 0.789-2.221; P≤0.0001) and in patient versus control groups (standard mean difference, 0.860; 95% CI, 0.402-1.318; P=0.0002; mean difference, 1.437; 95% CI, 0.426-2.448; P=0.0052). Subgroup analysis indicated differences in arterial stiffness between anthracycline-based and non-anthracycline-based therapies (standard mean difference, 0.20; 95% CI, 0.001-0.41; P=0.048), but not follow-up time. Conclusions Significant arterial stiffening occurs following anticancer therapy. Our findings support the use of arterial stiffness as part of a targeted vascular imaging strategy for the identification of early cardiovascular injury during treatment and for the detection of long-term cardiovascular injury into survivorship.
Keywords: arterial stiffness; cancer therapy; cardiotoxicity; pulse wave velocity; vascular toxicity.
Figures



Similar articles
-
Arterial Stiffness Use for Early Monitoring of Cardiovascular Adverse Events due to Anthracycline Chemotherapy in Breast Cancer Patients. A Pilot Study.Arq Bras Cardiol. 2018 Nov;111(5):721-728. doi: 10.5935/abc.20180168. Epub 2018 Sep 21. Arq Bras Cardiol. 2018. PMID: 30281690 Free PMC article.
-
Aortic wall stiffness as a side-effect of anti-cancer medication.Expert Rev Cardiovasc Ther. 2019 Nov;17(11):791-799. doi: 10.1080/14779072.2019.1691528. Epub 2019 Nov 20. Expert Rev Cardiovasc Ther. 2019. PMID: 31715108 Review.
-
Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment With Anthracycline-Containing Adjuvant Chemotherapy.Oncologist. 2016 Feb;21(2):141-9. doi: 10.1634/theoncologist.2015-0352. Epub 2016 Jan 13. Oncologist. 2016. PMID: 26764251 Free PMC article.
-
Increased arterial stiffness in children treated with anthracyclines for malignant disease.Coll Antropol. 2011 Jun;35(2):389-95. Coll Antropol. 2011. PMID: 21755708
-
Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials.Atherosclerosis. 2012 Mar;221(1):18-33. doi: 10.1016/j.atherosclerosis.2011.12.005. Epub 2011 Dec 9. Atherosclerosis. 2012. PMID: 22209214 Review.
Cited by
-
Time-Dependent Effect of Anthracycline-Based Chemotherapy on Central Arterial Stiffness: A Systematic Review and Meta-Analysis.Front Cardiovasc Med. 2022 Jul 5;9:873898. doi: 10.3389/fcvm.2022.873898. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35865379 Free PMC article.
-
Arterial effects of anthracycline: structural and inflammatory assessments in non-human primates and lymphoma patients using 18F-FDG positron emission tomography.bioRxiv [Preprint]. 2024 Jun 3:2024.05.30.596741. doi: 10.1101/2024.05.30.596741. bioRxiv. 2024. Update in: Clin Sci (Lond). 2025 Jan 15;139(1):29-41. doi: 10.1042/CS20241529. PMID: 38895275 Free PMC article. Updated. Preprint.
-
Tumor Necrosis Factor Alpha-Mediated Inflammation and Remodeling of the Extracellular Matrix Underlies Aortic Stiffening Induced by the Common Chemotherapeutic Agent Doxorubicin.Hypertension. 2021 May 5;77(5):1581-1590. doi: 10.1161/HYPERTENSIONAHA.120.16759. Epub 2021 Mar 15. Hypertension. 2021. PMID: 33719511 Free PMC article.
-
Anthracycline chemotherapy, vascular dysfunction and cognitive impairment: burgeoning topics and future directions.Future Cardiol. 2023 Sep;19(11):547-566. doi: 10.2217/fca-2022-0086. Epub 2022 Nov 10. Future Cardiol. 2023. PMID: 36354315 Free PMC article. Review.
-
Anthracycline chemotherapy-mediated vascular dysfunction as a model of accelerated vascular aging.Aging Cancer. 2021 Jun;2(1-2):45-69. doi: 10.1002/aac2.12033. Epub 2021 Jun 22. Aging Cancer. 2021. PMID: 34212156 Free PMC article.
References
-
- Mizia‐Stec K, Goscinska A, Mizia M, Haberka M, Chmiel A, Poborski W, Gasior Z. Anthracycline chemotherapy impairs the structure and diastolic function of the left ventricle and induces negative arterial remodelling. Kardiol Pol. 2013;71:681–690. - PubMed
-
- Frye JN, Sutterfield SL, Caldwell JT, Behnke BJ, Copp SW, Banister HR, Ade CJ. Vascular and autonomic changes in adult cancer patients receiving anticancer chemotherapy. J Appl Physiol (1985). 2018;125:198–204. - PubMed
-
- Vasiliadis I, Kolovou G, Mikhailidis DP. Cardiotoxicity and cancer therapy. Angiology. 2014;65:369–371. - PubMed
-
- Sutterfield SL, Caldwell JT, Post HK, Lovoy GM, Banister HR, Ade CJ. Lower cutaneous microvascular reactivity in adult cancer patients receiving chemotherapy. J Appl Physiol (1985). 2018;125:1141–1149. - PubMed
-
- Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

