EZH2 in Myeloid Malignancies
- PMID: 32650416
- PMCID: PMC7407223
- DOI: 10.3390/cells9071639
EZH2 in Myeloid Malignancies
Abstract
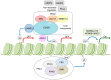
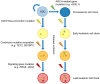
Our understanding of the significance of epigenetic dysregulation in the pathogenesis of myeloid malignancies has greatly advanced in the past decade. Enhancer of Zeste Homolog 2 (EZH2) is the catalytic core component of the Polycomb Repressive Complex 2 (PRC2), which is responsible for gene silencing through trimethylation of H3K27. EZH2 dysregulation is highly tumorigenic and has been observed in various cancers, with EZH2 acting as an oncogene or a tumor-suppressor depending on cellular context. While loss-of-function mutations of EZH2 frequently affect patients with myelodysplastic/myeloproliferative neoplasms, myelodysplastic syndrome and myelofibrosis, cases of chronic myeloid leukemia (CML) seem to be largely characterized by EZH2 overexpression. A variety of other factors frequently aberrant in myeloid leukemia can affect PRC2 function and disease pathogenesis, including Additional Sex Combs Like 1 (ASXL1) and splicing gene mutations. As the genetic background of myeloid malignancies is largely heterogeneous, it is not surprising that EZH2 mutations act in conjunction with other aberrations. Since EZH2 mutations are considered to be early events in disease pathogenesis, they are of therapeutic interest to researchers, though targeting of EZH2 loss-of-function does present unique challenges. Preliminary research indicates that combined tyrosine kinase inhibitor (TKI) and EZH2 inhibitor therapy may provide a strategy to eliminate the residual disease burden in CML to allow patients to remain in treatment-free remission.
Keywords: ASXL1; CML; EZH2; MDS; MPN; PRC2; mutations; myeloid malignancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

