Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System
- PMID: 32650422
- PMCID: PMC7552727
- DOI: 10.3390/bioengineering7030073
Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System
Abstract
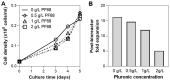
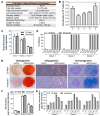
Human mesenchymal stem/stromal cells (hMSCs) have been investigated and proven to be a well-tolerated, safe therapy for a variety of indications, as shown by over 900 registered hMSC-based clinical trials. To meet the commercial demand for clinical manufacturing of hMSCs, production requires a scale that can achieve a lot size of ~100B cells, which requires innovative manufacturing technologies such as 3D bioreactors. A robust suspension bioreactor process that can be scaled-up to the relevant scale is therefore crucial. In this study, we developed a fed-batch, microcarrier-based bioreactor process, which enhances media productivity and drives a cost-effective and less labor-intensive hMSC expansion process. We determined parameter settings for various stages of the culture: inoculation, bioreactor culture, and harvest. Addition of a bioreactor feed, using a fed-batch approach, was necessary to replenish the mitogenic factors that were depleted from the media within the first 3 days of culture. Our study resulted in an optimized hMSC culture protocol that consistently achieved hMSC densities between 2 × 105-6 × 105 cells/mL within 5 days with no media exchange, maintaining the final cell population doubling level (PDL) at 16-20. Using multiple hMSC donors, we showed that this process was robust and yielded hMSCs that maintained expansion, phenotypic characteristic, and functional properties. The developed process in a vertical-wheel suspension bioreactor can be scaled to the levels needed to meet commercial demand of hMSCs.
Keywords: bioprocess; bioreactor; hMSCs; microcarrier.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rowley J.A. Meeting Lot-Size Challenges of Manufacturing Adherent Cells for Therapy. Bioprocess. Int. 2012;10:16–22.
-
- Schnitzler A.C., Kehoe D., Simler J., DiLeo A., Ball A. Scale-up of Human Mesenchymal Stem Cells on Microcarriers in Suspension in a Single-use Bioreactor. Bioprocess. Int. 2012;25:28–39.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

