Traumatic Stress Induces Prolonged Aggression Increase through Synaptic Potentiation in the Medial Amygdala Circuits
- PMID: 32651265
- PMCID: PMC7385664
- DOI: 10.1523/ENEURO.0147-20.2020
Traumatic Stress Induces Prolonged Aggression Increase through Synaptic Potentiation in the Medial Amygdala Circuits
Abstract
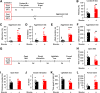
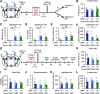
Traumatic stress can lead to heightened aggression which may be a symptom of psychiatric diseases such as PTSD and intermittent explosive disorder. The medial amygdala (MeA) is an evolutionarily conserved subnucleus of the amygdala that regulates attack behavior and behavioral responses to stressors. The precise contribution of the MeA in traumatic stress-induced aggression, however, requires further elucidation. In this study, we used foot shock to induce traumatic stress in mice and examine the mechanisms of prolonged aggression increase associated with it. Foot shock causes a prolonged increase in aggression that lasts at least one week. In vivo electrophysiological recordings revealed that foot shock induces potentiation of synapses formed between the MeA and the ventromedial hypothalamus (VmH) and bed nucleus of the stria terminalis (BNST). This synaptic potentiation lasts at least one week. Induction of synaptic depotentiation with low-frequency photostimulation (LFPS) immediately after foot shock suppresses the prolonged aggression increase without affecting non-aggressive social behavior, anxiety-like and depression-like behaviors, or fear learning. These results show that potentiation of the MeA-VmH and MeA-BNST circuits is essential for traumatic stress to cause a prolonged increase in aggression. These circuits may be potential targets for the development of therapeutic strategies to treat the aggression symptom associated with psychiatric diseases.
Keywords: PTSD; aggression; medial amygdala; synaptic plasticity; traumatic stress.
Copyright © 2020 Nordman et al.
Figures



References
-
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, Ed 5 Washington, DC: American Psychiatric Association.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
