Optogenetic Techniques for Manipulating and Sensing G Protein-Coupled Receptor Signaling
- PMID: 32651908
- PMCID: PMC9707295
- DOI: 10.1007/978-1-0716-0755-8_2
Optogenetic Techniques for Manipulating and Sensing G Protein-Coupled Receptor Signaling
Abstract
G protein-coupled receptors (GPCRs) form the largest class of membrane receptors in the mammalian genome with nearly 800 human genes encoding for unique subtypes. Accordingly, GPCR signaling is implicated in nearly all physiological processes. However, GPCRs have been difficult to study due in part to the complexity of their function which can lead to a plethora of converging or diverging downstream effects over different time and length scales. Classic techniques such as pharmacological control, genetic knockout and biochemical assays often lack the precision required to probe the functions of specific GPCR subtypes. Here we describe the rapidly growing set of optogenetic tools, ranging from methods for optical control of the receptor itself to optical sensing and manipulation of downstream effectors. These tools permit the quantitative measurements of GPCRs and their downstream signaling with high specificity and spatiotemporal precision.
Keywords: Calcium; G protein; G protein-coupled receptors (GPCR); LOV domain; Optogenetics; Photopharmacology.
Figures
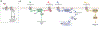
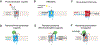


References
-
- Agnetta L, Kauk M, Canizal MCA, Messerer R, Holzgrabe U, Hoffmann C, and Decker M (2017). A Photoswitchable Dualsteric Ligand Controlling Receptor Efficacy. Angew Chem Int Ed Engl 56, 7282–7287. - PubMed
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, and Deisseroth K (2009). Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029. - PubMed
-
- Allen MD, and Zhang J (2006). Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun 348, 716–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

