Efficacy and safety of PT20, an iron-based phosphate binder, for the treatment of hyperphosphataemia: a randomized, double-blind, placebo-controlled, dose-ranging, Phase IIb study in patients with haemodialysis-dependent chronic kidney disease
- PMID: 32651955
- PMCID: PMC8311578
- DOI: 10.1093/ndt/gfaa116
Efficacy and safety of PT20, an iron-based phosphate binder, for the treatment of hyperphosphataemia: a randomized, double-blind, placebo-controlled, dose-ranging, Phase IIb study in patients with haemodialysis-dependent chronic kidney disease
Abstract
Background: Hyperphosphataemia is a common complication of chronic kidney disease (CKD). PT20 (ferric iron oxide adipate) is an investigational molecule engineered to offer enhanced phosphate-binding properties relative to other phosphate binders.
Methods: In this double-blind, parallel-group, placebo-controlled, dose-ranging study (ClinicalTrials.gov identifier NCT02151643), the efficacy and safety of 28 days of oral PT20 treatment were evaluated in patients with dialysis-dependent CKD. Participants were randomly assigned in an 8:8:8:13:13 ratio to receive PT20 (400, 800, 1600 or 3200 mg) or placebo three times daily.
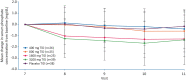
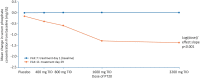
Results: Among 153 participants, 129 completed treatment [7 discontinued because of adverse events (AEs), 2 because of hyperphosphataemia and 15 for other reasons]. PT20 treatment for 28 days resulted in a statistically significant and dose-dependent reduction in serum phosphate concentration. There were no statistically significant effects of PT20 treatment on changes in haemoglobin or ferritin concentrations or transferrin saturation between Days 1 and 29. The incidence of treatment-emergent AEs was broadly similar across the PT20 and placebo groups (42-59% versus 44%). The most common PT20 treatment-related AEs were gastrointestinal, primarily diarrhoea (13-18%) and discoloured faeces (3-23%). No serious AEs were considered to be related to study treatment. There were no clinically significant changes in laboratory results reflecting acid/base status or increases in ferritin that could indicate the absorption of components of PT20.
Conclusions: In this first study investigating the efficacy and safety of PT20 in patients with hyperphosphataemia and dialysis-dependent CKD, PT20 significantly lowered serum phosphate concentrations and was generally well tolerated.
Keywords: PT20; chronic kidney disease; ferric oxide; hyperphosphataemia; phosphate binder.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved.
Figures

References
-
- Block GA, Hulbert-Shearon TE, Levin NW. et al.. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 - PubMed
-
- Kestenbaum B. Phosphate metabolism in the setting of chronic kidney disease: significance and recommendations for treatment. Semin Dial 2007; 20: 286–294 - PubMed
-
- Port FK, Pisoni RL, Bommer J. et al.. Improving outcomes for dialysis patients in the International Dialysis Outcomes and Practice Patterns study. Clin J Am Soc Nephrol 2006; 1: 246–255 - PubMed
-
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(4 Suppl 3): S1–S201 - PubMed

