Reconstruction of the Carbohydrate 6-O Sulfotransferase Gene Family Evolution in Vertebrates Reveals Novel Member, CHST16, Lost in Amniotes
- PMID: 32652010
- PMCID: PMC7353957
- DOI: 10.1093/gbe/evz274
Reconstruction of the Carbohydrate 6-O Sulfotransferase Gene Family Evolution in Vertebrates Reveals Novel Member, CHST16, Lost in Amniotes
Abstract
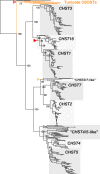
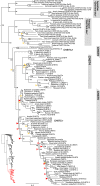
Glycosaminoglycans are sulfated polysaccharide molecules, essential for many biological processes. The 6-O sulfation of glycosaminoglycans is carried out by carbohydrate 6-O sulfotransferases (C6OSTs), previously named Gal/GalNAc/GlcNAc 6-O sulfotransferases. Here, for the first time, we present a detailed phylogenetic reconstruction, analysis of gene synteny conservation and propose an evolutionary scenario for the C6OST family in major vertebrate groups, including mammals, birds, nonavian reptiles, amphibians, lobe-finned fishes, ray-finned fishes, cartilaginous fishes, and jawless vertebrates. The C6OST gene expansion likely started early in the chordate lineage, giving rise to four ancestral genes after the divergence of tunicates and before the emergence of extant vertebrates. The two rounds of whole-genome duplication in early vertebrate evolution (1R/2R) only contributed two additional C6OST subtype genes, increasing the vertebrate repertoire from four genes to six, divided into two branches. The first branch includes CHST1 and CHST3 as well as a previously unrecognized subtype, CHST16 that was lost in amniotes. The second branch includes CHST2, CHST7, and CHST5. Subsequently, local duplications of CHST5 gave rise to CHST4 in the ancestor of tetrapods, and to CHST6 in the ancestor of primates. The teleost-specific gene duplicates were identified for CHST1, CHST2, and CHST3 and are result of whole-genome duplication (3R) in the teleost lineage. We could also detect multiple, more recent lineage-specific duplicates. Thus, the vertebrate repertoire of C6OST genes has been shaped by gene duplications and gene losses at several stages of vertebrate evolution, with implications for the evolution of skeleton, nervous system, and cell-cell interactions.
Keywords: Gal/GalNAc/GlcNAc 6-O sulfotransferases; carbohydrate 6-O sulfotransferases; vertebrate; whole-genome duplication.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures





Similar articles
-
Dynamic evolution of transient receptor potential vanilloid (TRPV) ion channel family with numerous gene duplications and losses.Front Endocrinol (Lausanne). 2022 Nov 1;13:1013868. doi: 10.3389/fendo.2022.1013868. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387917 Free PMC article.
-
The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications.BMC Evol Biol. 2013 Nov 2;13:238. doi: 10.1186/1471-2148-13-238. BMC Evol Biol. 2013. PMID: 24180662 Free PMC article.
-
Whole Genome Duplications Shaped the Receptor Tyrosine Kinase Repertoire of Jawed Vertebrates.Genome Biol Evol. 2016 Jun 3;8(5):1600-13. doi: 10.1093/gbe/evw103. Genome Biol Evol. 2016. PMID: 27260203 Free PMC article.
-
New Insights Into the Evolutionary History of Melatonin Receptors in Vertebrates, With Particular Focus on Teleosts.Front Endocrinol (Lausanne). 2020 Sep 24;11:538196. doi: 10.3389/fendo.2020.538196. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33071966 Free PMC article. Review.
-
Evolution of endothelin receptors in vertebrates.Gen Comp Endocrinol. 2014 Dec 1;209:21-34. doi: 10.1016/j.ygcen.2014.06.028. Epub 2014 Jul 8. Gen Comp Endocrinol. 2014. PMID: 25010382 Review.
Cited by
-
Tissue-specific expression of carbohydrate sulfotransferases drives keratan sulfate biosynthesis in the notochord and otic vesicles of Xenopus embryos.Front Cell Dev Biol. 2023 Mar 14;11:957805. doi: 10.3389/fcell.2023.957805. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36998246 Free PMC article.
-
Towards complete and error-free genome assemblies of all vertebrate species.Nature. 2021 Apr;592(7856):737-746. doi: 10.1038/s41586-021-03451-0. Epub 2021 Apr 28. Nature. 2021. PMID: 33911273 Free PMC article.
References
-
- Abdullayev I, Kirkham M, Björklund ÅK, Simon A, Sandberg R.. 2013. A reference transcriptome and inferred proteome for the salamander Notophthalmus viridescens. Exp Cell Res. 319(8):1187–1197. - PubMed
-
- Akama TO, Misra AK, Hindsgaul O, Fukuda MN.. 2002. Enzymatic synthesis in vitro of the disulfated disaccharide unit of corneal keratan sulfate. J Biol Chem. 277(45):42505–42513. - PubMed
-
- Akama TO, et al. 2000. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet. 26(2):237–241. - PubMed
-
- Akama TO, et al. 2001. Human corneal GlcNAc 6-O-sulfotransferase and mouse intestinal GlcNAc 6-O-sulfotransferase both produce keratan sulfate. J Biol Chem. 276(19):16271–16278. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

