Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis
- PMID: 32652164
- PMCID: PMC7345400
- DOI: 10.1016/j.jinf.2020.07.008
Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis
Abstract
Background: As the novel SARS-CoV-2 pandemic occurred, no specific treatment was yet available. Inflammatory response secondary to viral infection might be the driver of severe diseases. We report the safety and efficacy (in terms of overall survival and hospital discharge) of the anti-IL6 tocilizumab (TCZ) in subjects with COVID-19.
Methods: This retrospective, single-center analysis included all the patients consecutively admitted to our Hospital with severe or critical COVID-19 who started TCZ treatment from March 13th to April 03rd, 2020. A 1:2 matching to patients not treated with TCZ was performed according to age, sex, severity of disease, P/F, Charlson Comorbidity Index and length of time between symptoms onset and hospital admittance. Descriptive statistics and non-parametric tests to compare the groups were applied. Kaplan Meier probability curves and Cox regression models for survival, hospital discharge and orotracheal intubation were used.
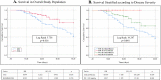
Results: Seventy-four patients treated with TCZ were matched with 148 matched controls. They were mainly males (81.5%), Caucasian (82.0%) and with a median age of 59 years. The majority (69.8%) showed critical stage COVID-19 disease. TCZ use was associated with a better overall survival (HR 0.499 [95% CI 0.262-0.952], p = 0.035) compared to controls but with a longer hospital stay (HR 1.658 [95% CI 1.088-2.524], p = 0.019) mainly due to biochemical, respiratory and infectious adverse events.
Discussion: TCZ use resulted potentially effective on COVID-19 in terms of overall survival. Caution is warranted given the potential occurrence of adverse events.
Financial support: Some of the tocilizumab doses used in the subjects included in this analysis were provided by the "Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia" (EudraCT Number: 2020-001110-38) supported by the Italian National Agency for Drugs (AIFA). No specific funding support was planned for study design, data collection and analysis and manuscript writing of this paper.
Keywords: COVID-19; IL-6; Orotracheal tube; SARS-CoV2; Tocilizumab.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest None.
Figures





Comment in
-
Preserved C-reactive protein responses to blood stream infections following tocilizumab treatment for COVID-19.J Infect. 2021 Nov;83(5):607-635. doi: 10.1016/j.jinf.2021.08.017. Epub 2021 Aug 14. J Infect. 2021. PMID: 34400218 Free PMC article. No abstract available.
-
Genetic variation of interleukin-1 receptor type 1 is associated with severity of COVID-19 disease.J Infect. 2022 Feb;84(2):e19-e21. doi: 10.1016/j.jinf.2021.12.010. Epub 2021 Dec 21. J Infect. 2022. PMID: 34952040 Free PMC article. No abstract available.
References
-
- European Center for Disease Prevention and Control. Available at:https://www.ecdc.europa.eu/en/covid-19-pandemic. Accessed on April 29th, 2020.
-
- Uciechowski P., Dempke W.C.M. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98:131–137. - PubMed
-
- Norelli M., Camisa B., Barbiera G. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

