Lack of sensitivity of an IVD/CE-labelled kit targeting the S gene for detection of SARS-CoV-2
- PMID: 32652240
- PMCID: PMC7345380
- DOI: 10.1016/j.cmi.2020.06.036
Lack of sensitivity of an IVD/CE-labelled kit targeting the S gene for detection of SARS-CoV-2
Abstract
Objectives: New molecular tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being rapidly launched in response to the coronavirus disease 2019 (COVID-19) pandemic. The aim of this study was to evaluate the analytical and clinical performance of the VIASURE SARS-CoV-2 S gene RT-PCR Kit on the BD Max™ system and to compare results with those obtained with the cobas® SARS-CoV-2 test on the cobas® 6800 system.
Methods: For testing the analytical performance, reference material was used. Clinical samples (n = 101) obtained from individuals with symptoms compatible with COVID-19 were studied. Oropharyngeal and nasopharyngeal swabs were collected by using either ESwab™ or UTM™ collection systems.
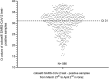
Results: When the analytical performance was evaluated, the sample containing the lowest SARS-CoV-2 concentration tested negative with the VIASURE test whereas results obtained with the cobas® test were found to be concordant with the results expected. Six out of the 101 clinical samples (5.9%) showed an inhibition with the VIASURE test. When analysing the remaining 95 clinical samples, 27 were found to be negative with both assays. Of 68 samples that were positive with the cobas® test, the VIASURE test missed 21 (30.9 %) samples. All of those 21 samples had shown Ct values ≥ 31 with the cobas® 6800 system. None of the samples tested positive with the VIASURE test and negative with the cobas® test.
Conclusions: The VIASURE test was impaired by a lack of sensitivity and a relatively high number of invalid results. When using the VIASURE test for routine testing, a significant number of COVID-19-positive samples would have been missed.
Keywords: COVID-19; Cobas; Diagnostics; Real-time RT-PCR; VIASURE.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
-
Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test Using Nasopharyngeal Swabs Transported in Viral Transport Media and Dry Nasal Swabs in a New York City Academic Institution.J Clin Microbiol. 2020 Jul 23;58(8):e01136-20. doi: 10.1128/JCM.01136-20. Print 2020 Jul 23. J Clin Microbiol. 2020. PMID: 32471894 Free PMC article.
-
Automated SARS-COV-2 RNA extraction from patient nasopharyngeal samples using a modified DNA extraction kit for high throughput testing.Ann Saudi Med. 2020 Sep-Oct;40(5):373-381. doi: 10.5144/0256-4947.2020.373. Epub 2020 Oct 1. Ann Saudi Med. 2020. PMID: 32954791 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
SARS-CoV-2 detection in different respiratory sites: A systematic review and meta-analysis.EBioMedicine. 2020 Sep;59:102903. doi: 10.1016/j.ebiom.2020.102903. Epub 2020 Jul 24. EBioMedicine. 2020. PMID: 32718896 Free PMC article.
Cited by
-
Reliable Diagnostics of SARS-CoV-2 Infections Using One- and Two-Gene Molecular Tests for a Viral RNA Detection-Results Questioning Previous Observations.Diagnostics (Basel). 2021 Oct 5;11(10):1839. doi: 10.3390/diagnostics11101839. Diagnostics (Basel). 2021. PMID: 34679537 Free PMC article.
-
Analytical and clinical comparison of Viasure (CerTest Biotec) and 2019-nCoV CDC (IDT) RT-qPCR kits for SARS-CoV2 diagnosis.Virology. 2021 Jan 15;553:154-156. doi: 10.1016/j.virol.2020.10.010. Epub 2020 Nov 18. Virology. 2021. PMID: 33278737 Free PMC article.
-
A Comparative Analysis of Molecular Biological Methods for the Detection of SARS-CoV-2 and Testing the In Vitro Infectivity of the Virus.Microorganisms. 2024 Jan 17;12(1):180. doi: 10.3390/microorganisms12010180. Microorganisms. 2024. PMID: 38258006 Free PMC article.
-
CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples.Biomed Pharmacother. 2021 Aug;140:111772. doi: 10.1016/j.biopha.2021.111772. Epub 2021 May 27. Biomed Pharmacother. 2021. PMID: 34062417 Free PMC article. Review.
-
Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: a systematic review and meta-analysis.Clin Microbiol Infect. 2021 Mar;27(3):341-351. doi: 10.1016/j.cmi.2020.11.002. Epub 2020 Nov 11. Clin Microbiol Infect. 2021. PMID: 33188933 Free PMC article.
References
-
- CerTest Biotec, S.L.; Zaragoza (Spain): 2020. Handbook BD REF 444212 VIASURE SARS-CoV-2 S-gene real time PCR detection kit.
-
- Knabl L., Grutsch I., Orth-Holler D. Comparison of the BD MAX® Enteric Bacterial Panel assay with conventional diagnostic procedures in diarrheal stool samples. Eur J Clin Microbiol Infect Dis. 2016;35:131–136. - PubMed
-
- Grimmond T., Neelakanta A., Miller B., Saiyed A., Gill P., Cadnum J. A microbiological study to investigate the carriage and transmission-potential of Clostridium difficile spores on single-use and reusable sharps containers. Am J Infect Control. 2018;46:1154–1159. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

