An integrated PK-PD model for cortisol and the 17-hydroxyprogesterone and androstenedione biomarkers in children with congenital adrenal hyperplasia
- PMID: 32652643
- PMCID: PMC9328191
- DOI: 10.1111/bcp.14470
An integrated PK-PD model for cortisol and the 17-hydroxyprogesterone and androstenedione biomarkers in children with congenital adrenal hyperplasia
Abstract
Aims: The aim of this study was to characterize the pharmacokinetic/pharmacodynamic relationships of cortisol and the adrenal biomarkers 17-hydroxyprogesterone and androstenedione in children with congenital adrenal hyperplasia (CAH).
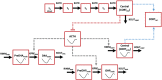
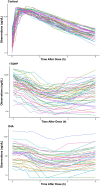
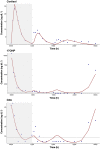
Methods: A nonlinear mixed-effect modelling approach was used to analyse cortisol, 17-hydroxyprogesterone and androstenedione concentrations obtained over 6 hours from children with CAH (n = 50). A circadian rhythm was evident and the model leveraged literature information on circadian rhythm in untreated children with CAH. Indirect response models were applied in which cortisol inhibited the production rate of all three compounds using an Imax model.
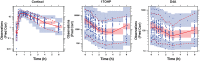
Results: Cortisol was characterized by a one-compartment model with apparent clearance and volume of distribution estimated at 22.9 L/h/70 kg and 41.1 L/70 kg, respectively. The IC50 values of cortisol concentrations for cortisol, 17-hydroxyprogesterone and androstenedione were estimated to be 1.36, 0.45 and 0.75 μg/dL, respectively. The inhibitory effect was found to be more potent on 17OHP than D4A, and the IC50 values were higher in salt-wasting subjects than simple virilizers. Production rates of cortisol, 17-hydroxyprogesterone and androstenedione were higher in simple-virilizer subjects. Half-lives of cortisol, 17-hydroxyprogesterone and androstenedione were 60, 47 and 77 minutes, respectively.
Conclusion: Rapidly changing biomarker responses to cortisol concentrations highlight that single measurements provide volatile information about a child's disease control. Our model closely captured observed cortisol, 17-hydroxyprogesterone and androstenedione concentrations. It can be used to predict concentrations over 24 hours and allows many novel exposure metrics to be calculated, e.g., AUC, AUC-above-threshold, time-within-range, etc. Our long-range goal is to uncover dose-exposure-outcome relationships that clinicians can use in adjusting hydrocortisone dose and timing.
Keywords: compartmental analysis; endocrinology; mathematical modelling; paediatric; population pharmacokinetics-pharmacodynamics; steroids.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
There are no competing interests to declare.
Figures
References
-
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty‐four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33(1):14‐22. - PubMed
-
- Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539‐2549. - PubMed
-
- Veldhuis JD, Iranmanesh A, Lizarralde G, Johnson ML. Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol. 1989;257:E6‐E14. - PubMed
-
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261(5 Pt 2):R1257‐R1268. - PubMed
-
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76(6):1505‐1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

