Controlling Mirror Symmetry Breaking and Network Formation in Liquid Crystalline Cubic, Isotropic Liquid and Crystalline Phases of Benzil-Based Polycatenars
- PMID: 32652801
- PMCID: PMC7756378
- DOI: 10.1002/chem.202002869
Controlling Mirror Symmetry Breaking and Network Formation in Liquid Crystalline Cubic, Isotropic Liquid and Crystalline Phases of Benzil-Based Polycatenars
Abstract
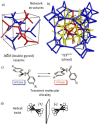
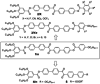
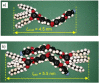
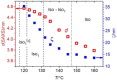
Spontaneous development of chirality in systems composed of achiral molecules is important for new routes to asymmetric synthesis, chiral superstructures and materials, as well as for the understanding of the mechanisms of emergence of prebiotic chirality. Herein, it is shown that the 4,4'-diphenylbenzil unit is a universal transiently chiral bent building block for the design of multi-chained (polycatenar) rod-like molecules capable of forming a wide variety of helically twisted network structures in the liquid, the liquid crystalline (LC) and the crystalline state. Single polar substituents at the apex of tricatenar molecules support the formation of the achiral (racemic) cubic double network phase with Ia d symmetry and relatively small twist along the networks. The combination of an alkyl chain with fluorine substitution leads to the homogeneously chiral triple network phase with I23 space group, and in addition, provides a mirror symmetry broken liquid. Replacing F by Cl or Br further increases the twist, leading to a short pitch double gyroid Ia d phase, which is achiral again. The effects of the structural variations on the network structures, either leading to achiral phases or chiral conglomerates are analyzed.
Keywords: chirality; cubic phases; liquid crystals; mirror symmetry breaking; soft matter.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Mirror Symmetry Breaking and Network Formation in Achiral Polycatenars with Thioether Tail.Chemistry. 2021 Oct 25;27(60):14921-14930. doi: 10.1002/chem.202102226. Epub 2021 Oct 7. Chemistry. 2021. PMID: 34542201 Free PMC article.
-
Switching Chirophilic Self-assembly: From meso-structures to Conglomerates in Liquid and Liquid Crystalline Network Phases of Achiral Polycatenar Compounds.Chemistry. 2022 Dec 1;28(67):e202201857. doi: 10.1002/chem.202201857. Epub 2022 Oct 5. Chemistry. 2022. PMID: 35866649 Free PMC article.
-
Spontaneous mirror symmetry breaking in benzil-based soft crystalline, cubic liquid crystalline and isotropic liquid phases.Chem Sci. 2020 Apr 30;11(23):5902-5908. doi: 10.1039/d0sc01396j. eCollection 2020 Jun 21. Chem Sci. 2020. PMID: 32874512 Free PMC article.
-
Mirror Symmetry Breaking by Chirality Synchronisation in Liquids and Liquid Crystals of Achiral Molecules.Chemphyschem. 2016 Jan 4;17(1):9-26. doi: 10.1002/cphc.201500601. Epub 2015 Oct 13. Chemphyschem. 2016. PMID: 26416335 Review.
-
Spontaneous achiral symmetry breaking in liquid crystalline phases.Top Curr Chem. 2012;318:303-30. doi: 10.1007/128_2011_242. Top Curr Chem. 2012. PMID: 21915774 Review.
Cited by
-
Mirror Symmetry Breaking and Network Formation in Achiral Polycatenars with Thioether Tail.Chemistry. 2021 Oct 25;27(60):14921-14930. doi: 10.1002/chem.202102226. Epub 2021 Oct 7. Chemistry. 2021. PMID: 34542201 Free PMC article.
-
Switching Chirophilic Self-assembly: From meso-structures to Conglomerates in Liquid and Liquid Crystalline Network Phases of Achiral Polycatenar Compounds.Chemistry. 2022 Dec 1;28(67):e202201857. doi: 10.1002/chem.202201857. Epub 2022 Oct 5. Chemistry. 2022. PMID: 35866649 Free PMC article.
-
Bicontinuous Gyroid Phase of a Water-Swollen Wedge-Shaped Amphiphile: Studies with In-Situ Grazing-Incidence X-ray Scattering and Atomic Force Microscopy.Materials (Basel). 2021 May 28;14(11):2892. doi: 10.3390/ma14112892. Materials (Basel). 2021. PMID: 34071178 Free PMC article.
References
-
- Gasteiger J., Zupan J., Angew. Chem. Int. Ed. Engl. 1993, 32, 503–527;
- Angew. Chem. 1993, 105, 510–536.
-
- Kauffman S., At Home in the Universe, Oxford University Press, Oxford, 1995, pp. 54–58.
-
- None
-
- Otto S., Acc. Chem. Res. 2012, 45, 2200–2210; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

