Sotrastaurin, a PKC inhibitor, attenuates RANKL-induced bone resorption and attenuates osteochondral pathologies associated with the development of OA
- PMID: 32652826
- PMCID: PMC7412701
- DOI: 10.1111/jcmm.15404
Sotrastaurin, a PKC inhibitor, attenuates RANKL-induced bone resorption and attenuates osteochondral pathologies associated with the development of OA
Abstract
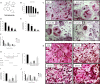
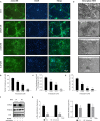
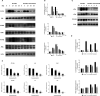
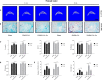
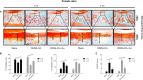
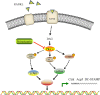
Osteoarthritis (OA) is a common degenerative disease that affects the musculoskeletal structure of the whole joint, which is characterized by progressive destruction of both articular cartilage and subchondral bone. Treatment of the bone pathologies, particularly osteoclast-mediated subchondral bone loss in the early stages of OA, could prevent subsequent cartilage degeneration and progression of OA. In the present study, the PKC inhibitor, Sotrastaurin, was found to inhibit RANKL-induced osteoclast formation in vitro in a dose- and time-dependent manner. In particular, SO exerted its anti-osteoclastic effect predominantly at the early stages of RANKL stimulation, suggesting inhibitory effects on precursor cell fusion. Using mature osteoclasts cultured on bovine bone discs, we showed that SO also exerts anti-resorptive effects on mature osteoclasts bone resorptive function. Mechanistically, SO attenuates the early activation of the p38, ERK and JNK signalling pathways, leeding to impaired induction of crucial osteoclast transcription factors c-Jun, c-Fos and NFATc1. We also showed that SO treatment significantly inhibited the phosphorylation of PKCδ and MARCKS, an upstream regulator of cathepsin K secretion. Finally, in animal studies, SO significantly alleviates the osteochondral pathologies of subchondral bone destruction as well as articular cartilage degeneration following DMM-induced OA, markedly improving OARSI scores. The reduced subchondral bone loss was associated with marked reductions in TRAP(+) osteoclasts in the subchondral bone tissue. Collectively, our data provide evidence for the protective effects of SO against OA by preventing aberrant subchondral bone and articular cartilage changes. Thus, SO demonstrates potential for further development as an alternative therapeutic option against OA.
Keywords: PKCδ; osteoclast; sotrastaurin; subchondral bone; therapeutics.
© The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interests to declare.
Figures
References
-
- Gu Y‐T, Chen J, Meng Z‐L, et al. Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother. 2017;93:1246‐1252. - PubMed
-
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323‐1330. - PubMed
-
- Iijima H, Aoyama T, Ito A, et al. Effects of short‐term gentle treadmill walking on subchondral bone in a rat model of instability‐induced osteoarthritis. Osteoarthritis Cartilage. 2015;23(9):1563‐1574. - PubMed
-
- Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state‐of‐the‐art, current prospects, and future challenges. Bone. 2016;85:81‐90. - PubMed
-
- Iijima H, Aoyama T, Ito A, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site‐specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22(7):1036‐1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

