A multi-level developmental approach to exploring individual differences in Down syndrome: genes, brain, behaviour, and environment
- PMID: 32653761
- PMCID: PMC7438975
- DOI: 10.1016/j.ridd.2020.103638
A multi-level developmental approach to exploring individual differences in Down syndrome: genes, brain, behaviour, and environment
Abstract
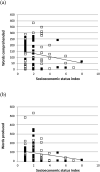
In this article, we focus on the causes of individual differences in Down syndrome (DS), exemplifying the multi-level, multi-method, lifespan developmental approach advocated by Karmiloff-Smith (1998, 2009, 2012, 2016). We evaluate the possibility of linking variations in infant and child development with variations in the (elevated) risk for Alzheimer's disease (AD) in adults with DS. We review the theoretical basis for this argument, considering genetics, epigenetics, brain, behaviour and environment. In studies 1 and 2, we focus on variation in language development. We utilise data from the MacArthur-Bates Communicative Development Inventories (CDI; Fenson et al., 2007), and Mullen Scales of Early Learning (MSEL) receptive and productive language subscales (Mullen, 1995) from 84 infants and children with DS (mean age 2;3, range 0;7 to 5;3). As expected, there was developmental delay in both receptive and expressive vocabulary and wide individual differences. Study 1 examined the influence of an environmental measure (socio-economic status as measured by parental occupation) on the observed variability. SES did not predict a reliable amount of the variation. Study 2 examined the predictive power of a specific genetic measure (apolipoprotein APOE genotype) which modulates risk for AD in adulthood. There was no reliable effect of APOE genotype, though weak evidence that development was faster for the genotype conferring greater AD risk (ε4 carriers), consistent with recent observations in infant attention (D'Souza, Mason et al., 2020). Study 3 considered the concerted effect of the DS genotype on early brain development. We describe new magnetic resonance imaging methods for measuring prenatal and neonatal brain structure in DS (e.g., volumes of supratentorial brain, cortex, cerebellar volume; Patkee et al., 2019). We establish the methodological viability of linking differences in early brain structure to measures of infant cognitive development, measured by the MSEL, as a potential early marker of clinical relevance. Five case studies are presented as proof of concept, but these are as yet too few to discern a pattern.
Keywords: Alzheimer’s disease; Down syndrome; apolipoprotein APOE gene; brain imaging; genetics; individual differences; socio-economic status; vocabulary development.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures





References
-
- Abbassi R., Johns T.G., Kassiou M. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. - PubMed
-
- Abbeduto L., Warren S.F., Conners F.A. Language development in Down syndrome: from the prelinguistic period to the acquisition of literacy. Ment Retard Dev Disabil Res Rev. 2007;13(3):247–261. - PubMed
-
- Antonarakis S.E., Lyle R., Dermitzakis E.T., Reymond A., Deutsch S. Chromosome 21 and Down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. - PubMed
-
- Aziz N.M., Guedj F., Pennings J.L., Olmos-Serrano J.L., Siegel A., Haydar T.F., Bianchi D.W. Lifespan analysis of brain development, gene expression and behavioral phenotypes in the Ts1Cje, Ts65Dn and Dp (16) 1/Yey mouse models of Down syndrome. Disease models & mechanisms. 2018 dmm-031013. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

