Neuropathy symptom and change: Inotersen treatment of hereditary transthyretin amyloidosis
- PMID: 32654156
- PMCID: PMC7540369
- DOI: 10.1002/mus.27023
Neuropathy symptom and change: Inotersen treatment of hereditary transthyretin amyloidosis
Abstract
Introduction: Hereditary transthyretin-mediated amyloidosis (hATTR) manifests as multisystem dysfunction, including progressive polyneuropathy. Inotersen, an antisense oligonucleotide, improved the course of neuropathic impairment in patients with hATTR in the pivotal NEURO-TTR study (NCT01737398). To determine inotersen's impact on symptoms and patients' neuropathy experience, we performed a post hoc analysis of the Neuropathy Symptoms and Change (NSC) score.
Methods: Stage 1 or 2 hATTR patients were randomized to receive weekly subcutaneous inotersen or placebo for 65 weeks. NSC score was assessed at baseline and 35 and 66 weeks.
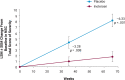
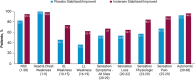
Results: At 66 weeks, inotersen-treated patients had symptom stabilization as compared with worsening in patients receiving placebo, based on total NSC score. There were also improvements in the subdomains of muscle weakness, sensory, pain, and autonomic symptoms, and for various individual items.
Discussion: Inotersen treatment stabilized neuropathy symptoms, including autonomic symptoms, in patients with hATTR according to NSC score. Thus, the NSC may be an effective measure to assess neuropathy progression and patients' neuropathy experience in clinical practice.
Keywords: Neuropathy Symptoms and Change; amyloidosis; hATTR; inotersen; transthyretin.
© 2020 The Authors. Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
P.J.B.D. has received honoraria/consultancy fees from Akcea. T.C. has received financial support to attend scientific meetings from Pfizer, Alnylam, and Biogen. M.W.C. has received honoraria/consultancy fees from NHI, Prothena, FoldRx, Ionis, Pfizer, Alnylam, PTC Therapeutics, and Genzyme for travel expenses related to presentations at medical meetings and for acting as a principal investigator in clinical trials. T.H.B. is on advisory boards for Akcea, Pfizer, and Alnylam; is a study investigator for Ionis and Alnylam; is a speaker for Alnylam; and has received speaker honoraria from Akcea. S.K. has received honoraria from Akcea and Alnylam. C.K. has served as a paid consultant for Akcea, Alnylam, Alexion, Biogen, CSL Behring, Cytokinetics, and Genzyme. J.L.B. has received honoraria from Ionis and Alnylam, and is a study investigator for Ionis, Alnylam, and Pfizer. M.J.P. has received honoraria from Pfizer and Alnylam. J.C.K. is a subinvestigator at the NEURO‐TTR treatment trial site and has received honoraria/consultancy fees from Akcea. J.F.W. is a subinvestigator at the NEURO‐TTR treatment trial site and has received consultancy fees from Ionis. W.J.L. has received grant/research support from Alnylam. M.L.M. has received research support from Ionis and Alnylam and has served as a consultant for Ionis. E.J.A. has received consultancy fees from Akcea. B.F.B. and S.W.J. are employees of Ionis. S.G. is an employee of Aurora Bio and former employee of Akcea. M.P. is an employee and shareholder of Akcea. P.J.D. has received financial support for the training of investigators for the conduct of therapeutic trials in hATTR with polyneuropathy from Ionis and Alnylam; he is also a consultant for Ionis and Alnylam.
Figures
References
-
- Benson MD, Kincaid JC. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve. 2007;36:411‐423. - PubMed
-
- Coelho T, Ericzon BG, Falk R, et al. A guide to transthyretin amyloidosis. Amyloidosis Foundation, Merrill Benson, Mathew Maurer. 2016. Last Accessed August 1, 2019. http://www.amyloidosis.org/wp-content/uploads/2017/05/2017-ATTR-guide.pdf.
-
- Ackermann EJ, Guo S, Booten S, et al. Clinical development of an antisense therapy for the treatment of transthyretin‐associated polyneuropathy. Amyloid. 2012;19(suppl 1):43‐44. - PubMed
-
- Benson MD, Waddington‐Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22‐31. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

