LPAR2 receptor activation attenuates radiation-induced disruption of apical junctional complexes and mucosal barrier dysfunction in mouse colon
- PMID: 32654268
- PMCID: PMC7725959
- DOI: 10.1096/fj.202000544R
LPAR2 receptor activation attenuates radiation-induced disruption of apical junctional complexes and mucosal barrier dysfunction in mouse colon
Abstract
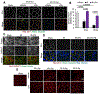
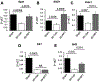
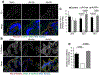
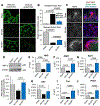
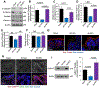
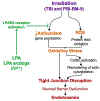
The tight junction (TJ) and barrier function of colonic epithelium is highly sensitive to ionizing radiation. We evaluated the effect of lysophosphatidic acid (LPA) and its analog, Radioprotein-1, on γ-radiation-induced colonic epithelial barrier dysfunction using Caco-2 and m-ICC12 cell monolayers in vitro and mice in vivo. Mice were subjected to either total body irradiation (TBI) or partial body irradiation (PBI-BM5). Intestinal barrier function was assessed by analyzing immunofluorescence localization of TJ proteins, mucosal inulin permeability, and plasma lipopolysaccharide (LPS) levels. Oxidative stress was analyzed by measuring protein thiol oxidation and antioxidant mRNA. In Caco-2 and m-ICC12 cell monolayers, LPA attenuated radiation-induced redistribution of TJ proteins, which was blocked by a Rho-kinase inhibitor. In mice, TBI and PBI-BM5 disrupted colonic epithelial tight junction and adherens junction, increased mucosal permeability, and elevated plasma LPS; TJ disruption by TBI was more severe in Lpar2-/- mice compared to wild-type mice. RP1, administered before or after irradiation, alleviated TBI and PBI-BM5-induced TJ disruption, barrier dysfunction, and endotoxemia accompanied by protein thiol oxidation and downregulation of antioxidant gene expression, cofilin activation, and remodeling of the actin cytoskeleton. These data demonstrate that LPAR2 receptor activation prevents and mitigates γ-irradiation-induced colonic mucosal barrier dysfunction and endotoxemia.
Keywords: endotoxemia; intestine; irradiation; lysophosphatidic acid; tight junction.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of interest
GT is a founder and stockholder in RxBio Inc. that has licensed the intellectual property for RP-1.
Figures



References
-
- Van Itallie CM, and Anderson JM (2006) Claudins and epithelial paracellular transport. Annu Rev Physiol 68, 403–429 - PubMed
-
- Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, and Rao RK (2003) Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem 278, 11916–11924 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

