Use of tocilizumab in kidney transplant recipients with COVID-19
- PMID: 32654422
- PMCID: PMC7405397
- DOI: 10.1111/ajt.16192
Use of tocilizumab in kidney transplant recipients with COVID-19
Abstract
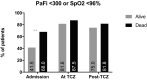
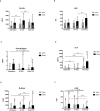
Acute respiratory distress syndrome associated with coronavirus infection is related to a cytokine storm with large interleukin-6 (IL-6) release. The IL-6-receptor blocker tocilizumab may control the aberrant host immune response in patients with coronavirus disease 2019 (COVID-19) . In this pandemic, kidney transplant (KT) recipients are a high-risk population for severe infection and showed poor outcomes. We present a multicenter cohort study of 80 KT patients with severe COVID-19 treated with tocilizumab during hospital admission. High mortality rate was identified (32.5%), related with older age (hazard ratio [HR] 3.12 for those older than 60 years, P = .039). IL-6 and other inflammatory markers, including lactic acid dehydrogenase, ferritin, and D-dimer increased early after tocilizumab administration and their values were higher in nonsurvivors. Instead, C-reactive protein (CRP) levels decreased after tocilizumab, and this decrease positively correlated with survival (mean 12.3 mg/L in survivors vs. 33 mg/L in nonsurvivors). Each mg/L of CRP soon after tocilizumab increased the risk of death by 1% (HR 1.01 [confidence interval 1.004-1.024], P = .003). Although patients who died presented with worse respiratory situation at admission, this was not significantly different at tocilizumab administration and did not have an impact on outcome in the multivariate analysis. Tocilizumab may be effective in controlling cytokine storm in COVID-19 but randomized trials are needed.
Keywords: clinical research/practice; infection and infectious agents - viral; kidney transplantation/nephrology; patient survival.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
References
-
- World Health Organization. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- Centro Nacional de Epidemiología, Instituto de Salud Carlos III. https://covid19.isciii.es/
-
- Organización Nacional de Trasplantes. http://www.ont.es/infesp/Memorias/Actividad_de_Donaci%C3%B3n_y_Trasplant...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

