The Current Hepatic Encephalopathy Pipeline
- PMID: 32655239
- PMCID: PMC7335727
- DOI: 10.1016/j.jceh.2020.01.001
The Current Hepatic Encephalopathy Pipeline
Abstract
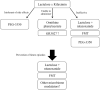
Hepatic encephalopathy (HE) is a complication of acute or chronic liver failure; its mechanism is complex, involving multiple organ systems, and is still being elucidated. The standard of care, lactulose, has remained generally unchanged for decades. However, in recent years, better understanding of the pathophysiology has yielded new therapeutic targets for this reversible condition. These novel treatments act both on traditional pathways established in the ammonia hypothesis and through more recently discovered mechanisms. Here, we review contemporary investigational therapies for HE. We used narrative reviews and searched ClinicalTrials.gov database for the condition "hepatic encephalopathy" through August 29, 2019. Our review yielded six key areas of therapeutic focus: (1) antibiotics against urease-producing gut bacteria, (2) intravenous ammonia scavengers, (3) modified synthetic probiotics, (4) fecal microbiota transplant, (5) brain steroid-modulating agents, and 6) nonlactulose laxatives. Active trials are ongoing in each of these therapeutic areas.
Keywords: CHESS, clinical hepatic encephalopathy staging scale; FMT, fecal microbiota transplant; HE, hepatic encephalopathy; HESA, hepatic encephalopathy scoring algorithm; MAD, multiple-ascending dose; MES, modified encephalopathy scale; ORT, object recognition test; PEG, polyethylene glycol-3350; SAD, single-ascending dose; SEV, saccadic eye velocity; ammonia; antibiotics; fecal microbiota transplant; hepatic encephalopathy; lactulose; therapy; trials.
© 2020 Indian National Association for Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
R.S.R. served on advisory boards for Mallinckrodt and has received research support from Valeant and Mallinckrodt (formerly Ocera Therapeutics). M.L.D. and A.J.R. have nothing to disclose.
Figures
References
-
- Frederick R.T. Current concepts in the pathophysiology and management of hepatic encephalopathy. Gastroenterol Hepatol (N Y). 2011;7:222–233. http://www.ncbi.nlm.nih.gov/pubmed/21857820 - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous