Subcortical Dopamine and Cognition in Schizophrenia: Looking Beyond Psychosis in Preclinical Models
- PMID: 32655348
- PMCID: PMC7325949
- DOI: 10.3389/fnins.2020.00542
Subcortical Dopamine and Cognition in Schizophrenia: Looking Beyond Psychosis in Preclinical Models
Abstract
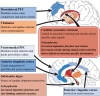
Schizophrenia is characterized by positive, negative and cognitive symptoms. All current antipsychotic treatments feature dopamine-receptor antagonism that is relatively effective at addressing the psychotic (positive) symptoms of schizophrenia. However, there is no clear evidence that these medications improve the negative or cognitive symptoms, which are the greatest predictors of functional outcomes. One of the most robust pathophysiological observations in patients with schizophrenia is increased subcortical dopamine neurotransmission, primarily in the associative striatum. This brain area has an important role in a range of cognitive processes. Dopamine is also known to play a major part in regulating a number of cognitive functions impaired in schizophrenia but much of this research has been focused on cortical dopamine. Emerging research highlights the strong influence subcortical dopamine has on a range of cognitive domains, including attention, reward learning, goal-directed action and decision-making. Nonetheless, the precise role of the associative striatum in the cognitive impairments observed in schizophrenia remains poorly understood, presenting an opportunity to revisit its contribution to schizophrenia. Without a better understanding of the mechanisms underlying cognitive dysfunction, treatment development remains at a standstill. For this reason, improved preclinical animal models are needed if we are to understand the complex relationship between subcortical dopamine and cognition. A range of new techniques are facillitating the discrete manipulation of dopaminergic neurotransmission and measurements of cognitive performance, which can be investigated using a variety of sensitive translatable tasks. This has the potential to aid the successful incorporation of recent clinical research to address the lack of treatment strategies for cognitive symptoms in schizophrenia. This review will give an overview on the current state of research focused on subcortical dopamine and cognition in the context of schizophrenia research. We also discuss future strategies and approaches aimed at improving the translational outcomes for the treatment of cognitive deficits in schizophrenia.
Keywords: goal-directed behavior; operant tasks; reversal learning; rodent; translation.
Copyright © 2020 Conn, Burne and Kesby.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

