Prevention of Neurite Spine Loss Induced by Dopamine D2 Receptor Overactivation in Striatal Neurons
- PMID: 32655360
- PMCID: PMC7324769
- DOI: 10.3389/fnins.2020.00642
Prevention of Neurite Spine Loss Induced by Dopamine D2 Receptor Overactivation in Striatal Neurons
Abstract
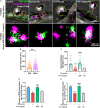
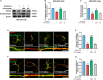
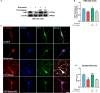
Psychosis has been considered a disorder of impaired neuronal connectivity. Evidence for excessive formation of dopamine D2 receptor (D2R) - disrupted in schizophrenia 1 (DISC1) complexes has led to a new perspective on molecular mechanisms involved in psychotic symptoms. Here, we investigated how excessive D2R-DISC1 complex formation induced by D2R agonist quinpirole affects neurite growth and dendritic spines in striatal neurons. Fluorescence resonance energy transfer (FRET), stochastic optical reconstruction microscopy (STORM), and cell penetrating-peptide delivery were used to study the cultured striatal neurons from mouse pups. Using these striatal neurons, our study showed that: (1) D2R interacted with DISC1 in dendritic spines, neurites and soma of cultured striatal neurons; (2) D2R and DISC1 complex accumulated in clusters in dendritic spines of striatal neurons and the number of the complex were reduced after application of TAT-D2pep; (3) uncoupling D2R-DISC1 complexes by TAT-D2pep protected neuronal morphology and dendritic spines; and (4) TAT-D2pep prevented neurite and dendritic spine loss, which was associated with restoration of expression levels of synaptophysin and PSD-95. In addition, we found that Neuropeptide Y (NPY) and GSK3β were involved in the protective effects of TAT-D2pep on the neurite spines of striatal spiny projection neurons. Thus, our results may offer a new strategy for precisely treating neurite spine deficits associated with schizophrenia.
Keywords: D2R–DISC1 complex; GABA; Neuropeptide Y; cell penetrating-peptide; schizophrenia; synaptic spine.
Copyright © 2020 Zheng, Su, Jin, Yu and Huang.
Figures



Similar articles
-
Uncoupling DISC1 × D2R Protein-Protein Interactions Facilitates Latent Inhibition in Disc1-L100P Animal Model of Schizophrenia and Enhances Synaptic Plasticity via D2 Receptors.Front Synaptic Neurosci. 2018 Sep 7;10:31. doi: 10.3389/fnsyn.2018.00031. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30245624 Free PMC article.
-
Aripiprazole and haloperidol protect neurite lesions via reducing excessive D2R-DISC1 complex formation.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun 8;92:59-69. doi: 10.1016/j.pnpbp.2018.12.007. Epub 2018 Dec 28. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30597182
-
Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through both D1 and D2 dopamine receptors.Neuropharmacology. 2013 Apr;67:432-43. doi: 10.1016/j.neuropharm.2012.11.030. Epub 2012 Dec 8. Neuropharmacology. 2013. PMID: 23231809
-
[When we have learned about the brain development from a disease-oriented study: DBZ regulates cortical cell positioning and neurite extension by sustaining the anterograde transport of Lis1/DISC1 through control of Ndel1 phosphorylation].Nihon Shinkei Seishin Yakurigaku Zasshi. 2016 Apr;36(2):43-50. Nihon Shinkei Seishin Yakurigaku Zasshi. 2016. PMID: 27333658 Review. Japanese.
-
Effects of antipsychotic drugs on neurites relevant to schizophrenia treatment.Med Res Rev. 2019 Jan;39(1):386-403. doi: 10.1002/med.21512. Epub 2018 May 22. Med Res Rev. 2019. PMID: 29785841 Review.
Cited by
-
Dopamine receptor D2 regulates GLUA1-containing AMPA receptor trafficking and central sensitization through the PI3K signaling pathway in a male rat model of chronic migraine.J Headache Pain. 2022 Aug 10;23(1):98. doi: 10.1186/s10194-022-01469-x. J Headache Pain. 2022. PMID: 35948867 Free PMC article.
-
Distinct molecular patterns in R6/2 HD mouse brain: Insights from spatiotemporal transcriptomics.Neuron. 2025 Aug 6;113(15):2416-2437.e6. doi: 10.1016/j.neuron.2025.05.014. Epub 2025 Jun 6. Neuron. 2025. PMID: 40482637
-
Canonical and Non-Canonical Antipsychotics' Dopamine-Related Mechanisms of Present and Next Generation Molecules: A Systematic Review on Translational Highlights for Treatment Response and Treatment-Resistant Schizophrenia.Int J Mol Sci. 2023 Mar 21;24(6):5945. doi: 10.3390/ijms24065945. Int J Mol Sci. 2023. PMID: 36983018 Free PMC article.
-
Dysregulated Signaling at Postsynaptic Density: A Systematic Review and Translational Appraisal for the Pathophysiology, Clinics, and Antipsychotics' Treatment of Schizophrenia.Cells. 2023 Feb 10;12(4):574. doi: 10.3390/cells12040574. Cells. 2023. PMID: 36831241 Free PMC article.
References
-
- Arnsten A. F., Cai J. X., Steere J. C., Goldman-Rakic P. S. (1995). Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J. Neurosci. 15(5 Pt 1) 3429–3439. 10.1523/jneurosci.15-05-03429.1995 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

