A Three-Dimensional Alzheimer's Disease Cell Culture Model Using iPSC-Derived Neurons Carrying A246E Mutation in PSEN1
- PMID: 32655369
- PMCID: PMC7325960
- DOI: 10.3389/fncel.2020.00151
A Three-Dimensional Alzheimer's Disease Cell Culture Model Using iPSC-Derived Neurons Carrying A246E Mutation in PSEN1
Abstract
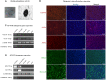
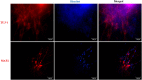
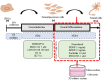
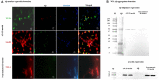
Alzheimer's disease (AD) is a chronic brain disorder characterized by progressive intellectual decline and memory and neuronal loss, caused mainly by extracellular deposition of amyloid-β (Aβ) and intracellular accumulation of hyperphosphorylated tau protein, primarily in areas implicated in memory and learning as prefrontal cortex and hippocampus. There are two forms of AD, a late-onset form that affects people over 65 years old, and the early-onset form, which is hereditable and affect people at early ages ~45 years. To date, there is no cure for the disease; consequently, it is essential to develop new tools for the study of processes implicated in the disease. Currently, in vitro AD three-dimensional (3D) models using induced pluripotent stem cells (iPSC)-derived neurons have broadened the horizon for in vitro disease modeling and gained interest for mechanistic studies and preclinical drug discovery due to their potential advantages in providing a better physiologically relevant information and more predictive data for in vivo tests. Therefore, this study aimed to establish a 3D cell culture model of AD in vitro using iPSCs carrying the A246E mutation. We generated human iPSCs from fibroblasts from a patient with AD harboring the A246E mutation in the PSEN1 gene. Cell reprogramming was performed using lentiviral vectors with Yamanaka's factors (OSKM: Oct4, Sox2, Klf4, and c-Myc). The resulting iPSCs expressed pluripotency genes (such as Nanog and Oct4), alkaline phosphatase activity, and pluripotency stem cell marker expression, such as OCT4, SOX2, TRA-1-60, and SSEA4. iPSCs exhibited the ability to differentiate into neuronal lineage in a 3D environment through dual SMAD inhibition as confirmed by Nestin, MAP2, and Tuj1 neural marker expression. These iPSC-derived neurons harbored Aβ oligomers confirmed by Western Blot (WB) and immunostaining. With human iPSC-derived neurons able to produce Aβ oligomers, we established a novel human hydrogel-based 3D cell culture model that recapitulates Aβ aggregation without the need for mutation induction or synthetic Aβ exposure. This model will allow the study of processes implicated in disease spread throughout the brain, the screening of molecules or compounds with therapeutic potential, and the development of personalized therapeutic strategies.
Keywords: 3D cell culture; Alzheimer’s disease; disease modeling; iPSC-derived neurons; personalized therapy.
Copyright © 2020 Hernández-Sapiéns, Reza-Zaldívar, Cevallos, Márquez-Aguirre, Gazarian and Canales-Aguirre.
Figures


Similar articles
-
Increased susceptibility to Aβ toxicity in neuronal cultures derived from familial Alzheimer's disease (PSEN1-A246E) induced pluripotent stem cells.Neurosci Lett. 2017 Feb 3;639:74-81. doi: 10.1016/j.neulet.2016.12.060. Epub 2016 Dec 26. Neurosci Lett. 2017. PMID: 28034781
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
Pathological manifestation of the induced pluripotent stem cell-derived cortical neurons from an early-onset Alzheimer's disease patient carrying a presenilin-1 mutation (S170F).Cell Prolif. 2020 Apr;53(4):e12798. doi: 10.1111/cpr.12798. Epub 2020 Mar 25. Cell Prolif. 2020. PMID: 32216003 Free PMC article.
-
3D culture models of Alzheimer's disease: a road map to a "cure-in-a-dish".Mol Neurodegener. 2016 Dec 9;11(1):75. doi: 10.1186/s13024-016-0139-7. Mol Neurodegener. 2016. PMID: 27938410 Free PMC article. Review.
-
Human iPSC application in Alzheimer's disease and Tau-related neurodegenerative diseases.Neurosci Lett. 2019 Apr 23;699:31-40. doi: 10.1016/j.neulet.2019.01.043. Epub 2019 Jan 24. Neurosci Lett. 2019. PMID: 30685408 Review.
Cited by
-
Human Dental Pulp Stem Cells Display a Potential for Modeling Alzheimer Disease-Related Tau Modifications.Front Neurol. 2021 Jan 25;11:612657. doi: 10.3389/fneur.2020.612657. eCollection 2020. Front Neurol. 2021. PMID: 33569035 Free PMC article.
-
Preconditioning of Mesenchymal Stem Cells Enhances the Neuroprotective Effects of Their Conditioned Medium in an Alzheimer's Disease In Vitro Model.Biomedicines. 2024 Oct 2;12(10):2243. doi: 10.3390/biomedicines12102243. Biomedicines. 2024. PMID: 39457556 Free PMC article.
-
Neuronal Dynamics and miRNA Signaling Differ between SH-SY5Y APPSwe and PSEN1 Mutant iPSC-Derived AD Models upon Modulation with miR-124 Mimic and Inhibitor.Cells. 2021 Sep 14;10(9):2424. doi: 10.3390/cells10092424. Cells. 2021. PMID: 34572073 Free PMC article.
-
Presenilin mutations and their impact on neuronal differentiation in Alzheimer's disease.Neural Regen Res. 2022 Jan;17(1):31-37. doi: 10.4103/1673-5374.313016. Neural Regen Res. 2022. PMID: 34100423 Free PMC article.
-
The occurrence and development of induced pluripotent stem cells.Front Genet. 2024 Apr 18;15:1389558. doi: 10.3389/fgene.2024.1389558. eCollection 2024. Front Genet. 2024. PMID: 38699229 Free PMC article. Review.
References
-
- Beers J., Gulbranson D. R., George N., Siniscalchi L. I., Jones J., Thomson J. A., et al. . (2012). Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat. Protoc. 7, 2029–2040. 10.1038/nprot.2012.130 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

