Long Noncoding RNA TCONS_00016406 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Regulating the miR-687/PTEN Pathway
- PMID: 32655407
- PMCID: PMC7325890
- DOI: 10.3389/fphys.2020.00622
Long Noncoding RNA TCONS_00016406 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Regulating the miR-687/PTEN Pathway
Abstract
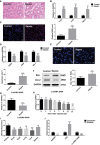
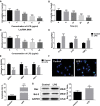
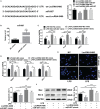
Acute kidney injury (AKI) is a common and serious complication of sepsis accompanied by kidney dysfunction resulting from various etiologies and pathophysiological processes. Unfortunately, there is currently no ideal therapeutic strategy for AKI. Numerous studies have confirmed that long noncoding RNAs (lncRNAs) play important regulatory roles in the pathogenesis of sepsis-associated AKI. In this study, lncRNA TCONS_00016406 (termed lncRNA 6406), a novel lncRNA identified by using TargetScan, was significantly downregulated in the kidney tissues of mice with sepsis-associated AKI. This study aimed to explore the role of lncRNA 6406 in lipopolysaccharide (LPS)-induced AKI and its potential molecular mechanism. The models of sepsis-induced AKI (called LPS-induced AKI models) in mice and cell lines were established with male C57BL/6 mice and renal tubular epithelial (PTEC) cells, respectively. Twenty-four hours after LPS administration, kidneys and cell samples were collected after various treatments to examine the alterations in the lncRNA 6406 levels and to evaluate the effects on LPS-induced inflammation, oxidative stress, and apoptosis through real-time PCR (RT-PCR) analysis, western blotting, and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. The results revealed that lncRNA 6406 could significantly attenuate LPS-induced AKI, as shown by the alleviation of inflammation, the suppression of oxidative stress and the inhibition of apoptosis. Mechanistically, a luciferase reporter assay and additional research showed that lncRNA 6406 functioned as a ceRNA to sponge miRNA-687, thereby modulating LPS-stimulated AKI by targeting the miR-687/PTEN axis; thus, this study presents a novel therapeutic strategy or sepsis-associated AKI.
Keywords: PTEN; acute kidney injury; long noncoding RNA 6406; miR-687; sepsis.
Copyright © 2020 Liu, Zhu, Zhang and Xu.
Figures



References
-
- Chawla L. S., Seneff M. G., Nelson D. R., Williams M., Levy H., Kimmel P. L., et al. . (2007). Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin. J. Am. Soc. Nephrol. 2, 22–30. 10.2215/CJN.02510706, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

