Modulation of the Inflammatory Response and Bone Healing
- PMID: 32655495
- PMCID: PMC7325942
- DOI: 10.3389/fendo.2020.00386
Modulation of the Inflammatory Response and Bone Healing
Abstract
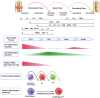
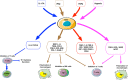
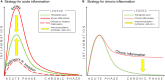
The optimal treatment for complex fractures and large bone defects is an important unsolved issue in orthopedics and related specialties. Approximately 5-10% of fractures fail to heal and develop non-unions. Bone healing can be characterized by three partially overlapping phases: the inflammatory phase, the repair phase, and the remodeling phase. Eventual healing is highly dependent on the initial inflammatory phase, which is affected by both the local and systemic responses to the injurious stimulus. Furthermore, immune cells and mesenchymal stromal cells (MSCs) participate in critical inter-cellular communication or crosstalk to modulate bone healing. Deficiencies in this inter-cellular exchange, inhibition of the natural processes of acute inflammation, and its resolution, or chronic inflammation due to a persistent adverse stimulus can lead to impaired fracture healing. Thus, an initial and optimal transient stage of acute inflammation is one of the key factors for successful, robust bone healing. Recent studies demonstrated the therapeutic potential of immunomodulation for bone healing by the preconditioning of MSCs to empower their immunosuppressive properties. Preconditioned MSCs (also known as "primed/ licensed/ activated" MSCs) are cultured first with pro-inflammatory cytokines (e.g., TNFα and IL17A) or exposed to hypoxic conditions to mimic the inflammatory environment prior to their intended application. Another approach of immunomodulation for bone healing is the resolution of inflammation with anti-inflammatory cytokines such as IL4, IL10, and IL13. In this review, we summarize the principles of inflammation and bone healing and provide an update on cellular interactions and immunomodulation for optimal bone healing.
Keywords: anti-inflammatory cytokines; bone healing; immunomodulation; inflammation; mesenchymal stromal cell; preconditioning; pro-inflammatory cytokines.
Copyright © 2020 Maruyama, Rhee, Utsunomiya, Zhang, Ueno, Yao and Goodman.
Figures
References
-
- Pahwa R, Jialal I. Chronic Inflammation. Internet: StatPearls Publishing; (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

