A new oral testosterone undecanoate therapy comes of age for the treatment of hypogonadal men
- PMID: 32655691
- PMCID: PMC7328356
- DOI: 10.1177/1756287220937232
A new oral testosterone undecanoate therapy comes of age for the treatment of hypogonadal men
Erratum in
-
Corrigendum to a new oral testosterone undecanoate (TU) therapy comes of age for the treatment of hypogonadal men.Ther Adv Urol. 2021 Aug 20;13:17562872211036391. doi: 10.1177/17562872211036391. eCollection 2021 Jan-Dec. Ther Adv Urol. 2021. PMID: 34434258 Free PMC article.
Abstract
Background: A novel formulation of oral testosterone undecanoate (TU) was studied in a long- and short-term phase III trial to evaluate safety and efficacy.
Methods: Hypogonadal men (age 18-65 years; two morning serum testosterone (T) <300 ng/dl with signs/symptoms) were recruited into a 365 day (trial I) or 105 day (trial II), randomized, multicenter trial. Patients were randomized 1:1 to oral TU (n = 161) or T-gel (n = 160) in trial I, and 3:1 to oral TU, twice daily (BID) JATENZO® (n = 166) or a topical T product [Axiron® (n = 56)] in trial II. Dose adjustments were based on average T concentrations (Cavg). Efficacy was assessed based on T levels, body composition and bone density. Safety was assessed by standard clinical measures.
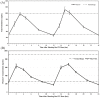
Results: Oral TU efficacy (% of patients with eugonadal T Cavg) was 84% (serum Cavg = 628 ± 343 ng/dl) and 87% (serum T equivalent Cavg ≈ 489 ± 155 ng/dl) in trials I and II, respectively. Oral TU significantly (p <0.0001) improved all Psychosexual Daily Questionnaire parameters in trials I and II. In trial I, lean mass increased 3.2 ± 2.7 kg and fat decreased by 2.4 ± 3.6 kg (both p <0.0001) and bone density improved in hip (+0.012 ± 0.0225 g/cm2) and spine (+0.018 ± 0.0422 g/cm2) after 365 days (both p <0.0001). Oral TU-associated adverse effects were consistent with other T-replacement therapies but oral TU patients experienced a greater number of mild gastrointestinal adverse effects. Oral TU subjects in both studies exhibited an increase in mean systolic blood pressure of about 3-5 mmHg. Oral TU was not associated with liver toxicity nor did it cause an elevation in high-sensitivity C-reactive protein or lipoprotein-associated phospholipase A2 (cardiovascular safety biomarkers) after 365 days of therapy.
Conclusion: A new oral TU formulation was safe and effective and represents a significant therapeutic advance for the treatment of appropriate hypogonadal men.
Keywords: male hypogonadism; testosterone; testosterone undecanoate.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: RSS served as principal investigator for studies CLAR-09007 (trial I) and CLAR-15012 (trial II) that were funded by Clarus Therapeutics, Inc. RSS has also received support from the NIH for testosterone studies in older men, and currently receives support from AbbVie as a study site for the Traverse Study of testosterone gel. He has also received support from NIH and NICHD for studies on male contraception where testosterone was a component of the experimental therapy. RED is employed by Clarus Therapeutics, Inc. (a private firm) and has an equity stake in the company.
Figures


References
-
- Nieschlag E, Nieschlag S. Endocrine history: the history of discovery, synthesis and development of testosterone for clinical use. Eur J Endocrinol 2019; 180: R201–R12. - PubMed
-
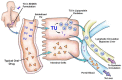
- Shackleford DM, Faassen WA, Houwing N, et al. Contribution of lymphatically transported testosterone undecanoate to the systemic exposure of testosterone after oral administration of two Andriol formulations in conscious lymph duct-cannulated dogs. J Pharmacol Exp Ther 2003; 306: 925–933. - PubMed
-
- Westaby D, Ogle SJ, Paradinas FJ, et al. Liver damage from long-term methyltestosterone. Lancet 1977; 2: 262–263. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

