Endothelial progenitor cells with stem cells enhance osteogenic efficacy
- PMID: 32655780
- PMCID: PMC7344071
Endothelial progenitor cells with stem cells enhance osteogenic efficacy
Abstract
Background: Mesenchymal stem cell (MSC)-based bone tissue engineering is a promising treatment option for maxillary sinus augmentation. Rapid vascularization is necessary to enhance the osteoinductive efficacy and prevent necrosis of the tissue-engineered bone. This study investigated whether the co-autotransplantation of endothelial progenitor cells (EPCs) could significantly enhance the in vivo osteogenic efficacy of MSCs and prevent necrosis of the tissue-engineered bone in a maxillary sinus augmentation model in dogs.
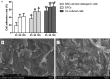
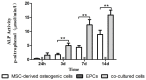
Methods: We evaluated the in vitro osteogenic activities of a clinically-used scaffold-deproteinized bovine bone (Bio-Oss) by examining cell adhesion and alkaline phosphatase (ALP) activity. In vivo, sinus augmentations were performed identically on both sides of dogs (n = 3 per group) using three treatment groups: (A) Bio-Oss with MSCs and EPCs; (B) Bio-Oss with MSCs; and (C) Bio-Oss with EPCs. The tissue implants were evaluated 24 weeks post-implantation.
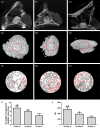
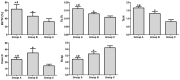
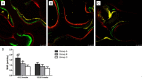
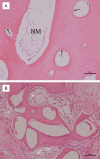
Results: In vitro, co-application of EPCs and MSCs on Bio-Oss significantly enhanced adhesion and ALP activity. In vivo, co-autotransplantation of MSCs and EPCs resulted in a significantly higher height, compressive strength, bone volume density, trabecular thickness, and trabecular number and a significantly lower trabecular separation compared with the other groups. The fluorescent test showed co-autotransplantation caused a significantly higher mineral apposition rate than the other groups. Histomorphometric analysis showed co-application resulted in the highest rate of new bone formation. Newly formed bone was frequently in the center of the implants with EPCs and MSCs, but not the other implants.
Conclusions: Co-autotransplantation of EPCs and MSCs significantly enhanced the in vivo osteogenic efficacy, suggesting promising potential for sinus augmentation.
Keywords: Endothelial progenitor cells; angiogenesis; bone marrow stromal cells; mesenchymal stem cells; osteogenesis; tissue-engineered bone.
AJTR Copyright © 2020.
Conflict of interest statement
None.
Figures


Similar articles
-
Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway.Stem Cell Res Ther. 2020 Dec 11;11(1):537. doi: 10.1186/s13287-020-02056-0. Stem Cell Res Ther. 2020. PMID: 33308309 Free PMC article.
-
Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration.Front Cell Dev Biol. 2021 May 13;9:674084. doi: 10.3389/fcell.2021.674084. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34079804 Free PMC article. Review.
-
Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: an in vitro study.Arch Med Res. 2013 Oct;44(7):504-13. doi: 10.1016/j.arcmed.2013.09.009. Epub 2013 Oct 10. Arch Med Res. 2013. PMID: 24120387
-
Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation.J Biomed Mater Res A. 2009 Sep 1;90(3):730-41. doi: 10.1002/jbm.a.32142. J Biomed Mater Res A. 2009. PMID: 18570318
-
Endothelial progenitors enhanced the osteogenic capacities of mesenchymal stem cells in vitro and in a rat alveolar bone defect model.Arch Oral Biol. 2016 Aug;68:123-30. doi: 10.1016/j.archoralbio.2016.04.007. Epub 2016 Apr 21. Arch Oral Biol. 2016. PMID: 27131592
Cited by
-
Effects of Yunnan Baiyao on the Differentiation of HPDLFs on the Bio-Oss® Collagen Scaffold in vivo.Int J Gen Med. 2022 Jun 3;15:5395-5405. doi: 10.2147/IJGM.S359921. eCollection 2022. Int J Gen Med. 2022. PMID: 35685694 Free PMC article.
-
Endothelial progenitor cells promote osteogenic differentiation in co-cultured with mesenchymal stem cells via the MAPK-dependent pathway.Stem Cell Res Ther. 2020 Dec 11;11(1):537. doi: 10.1186/s13287-020-02056-0. Stem Cell Res Ther. 2020. PMID: 33308309 Free PMC article.
-
Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds.Cells. 2022 Mar 8;11(6):926. doi: 10.3390/cells11060926. Cells. 2022. PMID: 35326377 Free PMC article.
-
Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats.Cells. 2021 Aug 31;10(9):2256. doi: 10.3390/cells10092256. Cells. 2021. PMID: 34571907 Free PMC article.
-
Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration.Front Cell Dev Biol. 2021 May 13;9:674084. doi: 10.3389/fcell.2021.674084. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34079804 Free PMC article. Review.
References
-
- Park JB. Use of cell-based approaches in maxillary sinus augmentation procedures. J Craniofac Surg. 2010;21:557–560. - PubMed
-
- Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the sinus consensus conference of 1996. Int J Oral Maxillofac Implants. 1998;13(Suppl):11–45. - PubMed
-
- Zizelmann C, Schoen R, Metzger MC, Schmelzeisen R, Schramm A, Dott B, Bormann KH, Gellrich NC. Bone formation after sinus augmentation with engineered bone. Clin Oral Implants Res. 2007;18:69–73. - PubMed
-
- Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants. 2009;24(Suppl):237–259. - PubMed
-
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21:696–710. - PubMed
LinkOut - more resources
Full Text Sources
