Improving the catalytic thermostability of Bacillus altitudinis W3 ω-transaminase by proline substitutions
- PMID: 32656056
- PMCID: PMC7324462
- DOI: 10.1007/s13205-020-02321-2
Improving the catalytic thermostability of Bacillus altitudinis W3 ω-transaminase by proline substitutions
Abstract
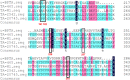
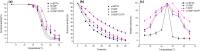
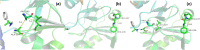
As a green biocatalyst, transaminase with high thermostability can be better employed to synthesize many pharmaceutical intermediates in industry. To improve the thermostability of (R)-selective amine transaminase from Bacillus altitudinis W3, related mutation sites were determined by multiple amino acid sequence alignment between wild-type ω-transaminase and four potential thermophilic ω-transaminases, followed by replacement of the related amino acid residues with proline by site-directed mutagenesis. Three stabilized mutants (D192P, T237P, and D192P/T237P) showing the highest stability were obtained and used for further analysis. Comparison with the wild-type enzyme revealed that the double mutant D192P/T237P exhibited the largest shift in thermostability, with a 2.5-fold improvement of t 1/2 at 40 °C, and a 6.3 °C increase in T 50 15, and a 5 °C higher optimal catalytic temperature. Additionally, this mutant exhibited an increase in catalytic efficiency (k cat/K m) relative to the wild-type enzyme. Modeling analysis indicated that the improved thermostability of the mutants could be associated with newly formed hydrophobic interactions and hydrogen bonds. This study shown that proline substitutions guided by sequence alignment to improve the thermostability of (R)-selective amine transaminase was effective and this method can also be used to engineering other enzymes.
Keywords: Amino transferase; Enzyme thermostability; Hydrogen bonds; Hydrophobic interactions; Proline substitutions; Protein engineering.
© King Abdulaziz City for Science and Technology 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures

Similar articles
-
[Deletion of a dynamic surface loop improves thermostability of (R)-selective amine transaminase from Aspergillus terreus].Sheng Wu Gong Cheng Xue Bao. 2017 Dec 25;33(12):1923-1933. doi: 10.13345/j.cjb.170279. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 29271170 Chinese.
-
Engineering thermostable (R)-selective amine transaminase from Aspergillus terreus through in silico design employing B-factor and folding free energy calculations.Biochem Biophys Res Commun. 2017 Jan 29;483(1):397-402. doi: 10.1016/j.bbrc.2016.12.131. Epub 2016 Dec 23. Biochem Biophys Res Commun. 2017. PMID: 28017723
-
Improving the Thermostability and Activity of Transaminase From Aspergillus terreus by Charge-Charge Interaction.Front Chem. 2021 Apr 14;9:664156. doi: 10.3389/fchem.2021.664156. eCollection 2021. Front Chem. 2021. PMID: 33937200 Free PMC article.
-
Improving thermostability of (R)-selective amine transaminase from Aspergillus terreus through introduction of disulfide bonds.Biotechnol Appl Biochem. 2018 Mar;65(2):255-262. doi: 10.1002/bab.1572. Epub 2017 Jul 17. Biotechnol Appl Biochem. 2018. PMID: 28639260
-
Construction of stabilized (R)-selective amine transaminase from Aspergillus terreus by consensus mutagenesis.J Biotechnol. 2019 Mar 10;293:8-16. doi: 10.1016/j.jbiotec.2019.01.007. Epub 2019 Jan 28. J Biotechnol. 2019. PMID: 30703468
Cited by
-
Tuning Thermostability and Catalytic Efficiency of Aflatoxin-Degrading Enzyme by Error-prone PCR.Appl Microbiol Biotechnol. 2023 Aug;107(15):4833-4843. doi: 10.1007/s00253-023-12610-4. Epub 2023 Jun 10. Appl Microbiol Biotechnol. 2023. PMID: 37300712
-
Improving the thermostability of GH49 dextranase AoDex by site-directed mutagenesis.AMB Express. 2023 Jan 19;13(1):7. doi: 10.1186/s13568-023-01513-2. AMB Express. 2023. PMID: 36656394 Free PMC article.
-
Computer-aided rational design strategy based on protein surface charge to improve the thermal stability of a novel esterase from Geobacillus jurassicus.Biotechnol Lett. 2024 Jun;46(3):443-458. doi: 10.1007/s10529-024-03473-4. Epub 2024 Mar 25. Biotechnol Lett. 2024. PMID: 38523202
-
Novel transaminases from thermophiles: from discovery to application.Microb Biotechnol. 2022 Jan;15(1):305-317. doi: 10.1111/1751-7915.13940. Epub 2021 Oct 29. Microb Biotechnol. 2022. PMID: 34713952 Free PMC article.
-
Exploring the Stability and Substrate Profile of Transaminase from Silicibacter pomeroyi with Ancestral Sequence Reconstruction.Chembiochem. 2025 Jul 11;26(13):e202500155. doi: 10.1002/cbic.202500155. Epub 2025 May 30. Chembiochem. 2025. PMID: 40279196 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

