The Neurovascular Unit in Glaucomatous Neurodegeneration
- PMID: 32656207
- PMCID: PMC7325980
- DOI: 10.3389/fcell.2020.00452
The Neurovascular Unit in Glaucomatous Neurodegeneration
Abstract
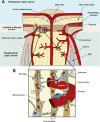
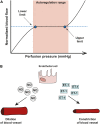
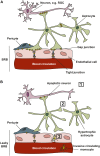
Glaucoma is a neurodegenerative disease of the visual system and leading cause of blindness worldwide. The disease is associated with sensitivity to intraocular pressure (IOP), which over a large range of magnitudes stresses retinal ganglion cell (RGC) axons as they pass through the optic nerve head in forming the optic projection to the brain. Despite clinical efforts to lower IOP, which is the only modifiable risk factor for glaucoma, RGC degeneration and ensuing loss of vision often persist. A major contributor to failure of hypotensive regimens is the multifactorial nature of how IOP-dependent stress influences RGC physiology and structure. This stress is conveyed to the RGC axon through interactions with structural, glial, and vascular components in the nerve head and retina. These interactions promote pro-degenerative pathways involving biomechanical, metabolic, oxidative, inflammatory, immunological and vascular challenges to the microenvironment of the ganglion cell and its axon. Here, we focus on the contribution of vascular dysfunction and breakdown of neurovascular coupling in glaucoma. The vascular networks of the retina and optic nerve head have evolved complex mechanisms that help to maintain a continuous blood flow and supply of metabolites despite fluctuations in ocular perfusion pressure. In healthy tissue, autoregulation and neurovascular coupling enable blood flow to stay tightly controlled. In glaucoma patients evidence suggests these pathways are dysfunctional, thus highlighting a potential role for pathways involved in vascular dysfunction in progression and as targets for novel therapeutic intervention.
Keywords: gap junctions; glaucoma; neurodegeneration; neurovascular coupling; neurovascular unit; vasculature.
Copyright © 2020 Wareham and Calkins.
Figures

References
-
- Alarcon-Martinez L., Yilmaz-Ozcan S., Yemisci M., Schallek J., Kilic K., Villafranca-Baughman D., et al. (2019). Retinal ischemia induces alpha-Sma-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 7:134. 10.1186/s40478-019-0761-z - DOI - PMC - PubMed
-
- Alm A., Bill A. (1973). Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp. Eye Res. 15 15–29. 10.1016/0014-4835(73)90185-1 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

