SARS-CoV-2 will constantly sweep its tracks: a vaccine containing CpG motifs in 'lasso' for the multi-faced virus
- PMID: 32656668
- PMCID: PMC7354743
- DOI: 10.1007/s00011-020-01377-3
SARS-CoV-2 will constantly sweep its tracks: a vaccine containing CpG motifs in 'lasso' for the multi-faced virus
Abstract
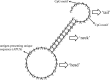
During the current COVID-19 pandemic, the global ratio between the dead and the survivors is approximately 1 to 10, which has put humanity on high alert and provided strong motivation for the intensive search for vaccines and drugs. It is already clear that if we follow the most likely scenario, which is similar to that used to create seasonal influenza vaccines, then we will need to develop improved vaccine formulas every year to control the spread of the new, highly mutable coronavirus SARS-CoV-2. In this article, using well-known RNA viruses (HIV, influenza viruses, HCV) as examples, we consider the main successes and failures in creating primarily highly effective vaccines. The experience accumulated dealing with the biology of zoonotic RNA viruses suggests that the fight against COVID-19 will be difficult and lengthy. The most effective vaccines against SARS-CoV-2 will be those able to form highly effective memory cells for both humoral (memory B cells) and cellular (cross-reactive antiviral memory T cells) immunity. Unfortunately, RNA viruses constantly sweep their tracks and perhaps one of the most promising solutions in the fight against the COVID-19 pandemic is the creation of 'universal' vaccines based on conservative SARS-CoV-2 genome sequences (antigen-presenting) and unmethylated CpG dinucleotides (adjuvant) in the composition of the phosphorothioate backbone of single-stranded DNA oligonucleotides (ODN), which can be effective for long periods of use. Here, we propose a SARS-CoV-2 vaccine based on a lasso-like phosphorothioate oligonucleotide construction containing CpG motifs and the antigen-presenting unique ACG-containing genome sequence of SARS-CoV-2. We found that CpG dinucleotides are the most rare dinucleotides in the genomes of SARS-CoV-2 and other known human coronaviruses, and hypothesized that their higher frequency could be responsible for the unwanted increased lethality to the host, causing a 'cytokine storm' in people who overexpress cytokines through the activation of specific Toll-like receptors in a manner similar to TLR9-CpG ODN interactions. Interestingly, the virus strains sequenced in China (Wuhan) in February 2020 contained on average one CpG dinucleotide more in their genome than the later strains from the USA (New York) sequenced in May 2020. Obviously, during the first steps of the microevolution of SARS-CoV-2 in the human population, natural selection tends to select viral genomes containing fewer CpG motifs that do not trigger a strong innate immune response, so the infected person has moderate symptoms and spreads SARS-CoV-2 more readily. However, in our opinion, unmethylated CpG dinucleotides are also capable of preparing the host immune system for the coronavirus infection and should be present in SARS-CoV-2 vaccines as strong adjuvants.
Keywords: COVID-19 pandemic; CpG motif; Phosphorothioate oligonucleotides; SARS-CoV-2; Vaccine.
Figures
References
-
- Kenney RT, Cross AS. Adjuvants for the future. In: Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro JP, Rappuoli R, editors. New Generation Vaccines. New York: Informa Healthcare USA, Inc.; 2010. pp. 250–262.
-
- Pulendran B, Powell J, Flavell RA. Modulating vaccine responses with innate immunity. In: Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro JP, Rappuoli R, editors. New Generation Vaccines. New York: Informa Healthcare USA, Inc.; 2010. pp. 183–190.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous