The entorhinal cortex of the monkey: VI. Organization of projections from the hippocampus, subiculum, presubiculum, and parasubiculum
- PMID: 32656783
- PMCID: PMC8933866
- DOI: 10.1002/cne.24983
The entorhinal cortex of the monkey: VI. Organization of projections from the hippocampus, subiculum, presubiculum, and parasubiculum
Abstract
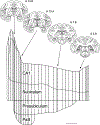
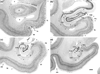
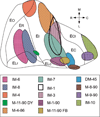
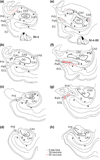
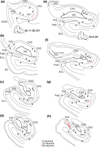
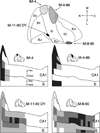
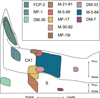
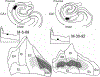
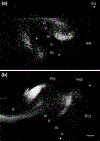
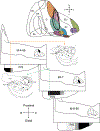
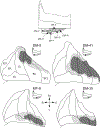
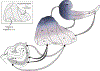
The organization of projections from the macaque monkey hippocampus, subiculum, presubiculum, and parasubiculum to the entorhinal cortex was analyzed using anterograde and retrograde tracing techniques. Projections exclusively originate in the CA1 field of the hippocampus and in the subiculum, presubiculum, and parasubiculum. The CA1 and subicular projections terminate most densely in Layers V and VI of the entorhinal cortex, with sparser innervation of the deep portion of Layers III and II. Entorhinal projections from CA1 and the subiculum are topographically organized such that a rostrocaudal axis of origin is related to a medial-to-lateral axis of termination. A proximodistal axis of origin in CA1 and distoproximal axis in subiculum are related to a rostrocaudal axis of termination in the entorhinal cortex. The presubiculum sends a dense, bilateral projection to caudal parts of the entorhinal cortex. This projection terminates most densely in Layer III with sparser termination in Layers I, II, and V. The same parts of entorhinal cortex receive a dense projection from the parasubiculum. This projection terminates in Layers III and II. Both presubicular and parasubicular projections demonstrate the same longitudinal topographic organization as the projections from CA1 and the subiculum. These studies demonstrate that: (a) hippocampal and subicular inputs to the entorhinal cortex in the monkey are organized similar to those described in nonprimate species; (b) the topographic organization of the projections from the hippocampus and subicular areas matches that of the reciprocal projections from the entorhinal cortex to the hippocampus and the subicular areas.
Keywords: hippocampal formation; memory; nonhuman primate; subiculum, presubiculum, parasubiculum.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors do not report a conflict of interest.
Figures





References
-
- Amaral DG, & Price JL (1983). An air pressure system for the injection of tracer substances into the brain. Journal of Neuroscience Methods, 9 (1), 35–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

