Advances and roadblocks in the treatment of malaria
- PMID: 32656850
- PMCID: PMC9437935
- DOI: 10.1111/bcp.14474
Advances and roadblocks in the treatment of malaria
Abstract
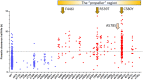
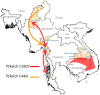
The deployment of artesunate for severe malaria and the artemisinin combination therapies (ACTs) for uncomplicated malaria has been a major advance in antimalarial therapeutics. These drugs have reduced treated mortality, accelerated recovery and reduced treatment failure rates and transmission from the treated infection. Artemisinin derivatives remain highly effective against falciparum malaria in most malaria endemic areas, but significant resistance has emerged in the Greater Mekong subregion of Southeast Asia. Resistance to artemisinins was followed by resistance to the ACT partner drugs, and fit multidrug resistant parasite lineages have now spread widely across the region. ACTs remain highly effective against P. vivax and the other malaria species. Recent studies have shown that radical curative regimens of primaquine (to prevent relapse) can be shortened to 7 days, and that the newly introduced single dose tafenoquine is an alternative, although the currently recommended dose is insufficient in Southeast Asia and Oceania. Targeted malaria elimination using focal mass treatments with dihydroartemisinin-piperaquine have proved safe and effective malaria elimination accelerators, but progress overall towards malaria elimination is slow. Indeed since 2015 overall malaria case numbers globally have risen. As new drugs will not become widely available in the near future, active measures to preserve the current antimalarials should be given the highest priority.
Keywords: antimalarial drugs; artemisinin; malaria; resistance.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures



References
-
- World Health Organization . Guidelines for the treatment of malaria. 3rd ed. Geneva: WHO; 2015.
-
- Fang ZJ (Ed). A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin). Houston, TX, USA: Translation Arnold K, Arnold M. Strategic Book Publishing & Rights Agency, LLC; 2013.
-
- Dondorp A, Nosten F, Stepniewska K, Day N, White NJ, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group . Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717‐725. - PubMed
-
- Artemether‐Quinine Meta‐analysis Study Group . A meta‐analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637‐650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

