Gastrointestinal organoids: a next-generation tool for modeling human development
- PMID: 32658619
- PMCID: PMC7509262
- DOI: 10.1152/ajpgi.00199.2020
Gastrointestinal organoids: a next-generation tool for modeling human development
Abstract
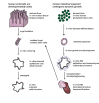
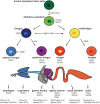
Gastrointestinal organoids are an exciting new tool for modeling human development, physiology, and disease in human tissue. Derived from pluripotent stem cells, gastrointestinal organoids consist of epithelial and mesenchymal cells organized in an intricate, three-dimensional structure that recapitulates the physiology and microscopic anatomy of the human gastrointestinal (GI) tract. In vitro derivation of gastrointestinal organoids from definitive endoderm has permitted an exploration of the complex signaling pathways required for the initial maturation of each individual gastrointestinal organ. Further maturation beyond an early fetal state currently requires transplantation into an immunocompromised host. Transplantation-induced maturation provides an opportunity to functionally interrogate the key mechanisms underlying development of the human GI tract. Gastrointestinal organoids can also be used to model human diseases and ultimately may serve as the basis for developing novel, personalized therapies for human intestinal diseases.
Keywords: disease modeling; enteroid; gastrointestinal development; human intestinal organoid; stem cells.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Berdon WE, Baker DH, Blanc WA, Gay B, Santulli TV, Donovan C. Megacystis-microcolon-intestinal hypoperistalsis syndrome: a new cause of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls. AJR Am J Roentgenol 126: 957–964, 1976. doi:10.2214/ajr.126.5.957. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

