Positron Emission Tomography-Directed Therapy for Patients With Limited-Stage Diffuse Large B-Cell Lymphoma: Results of Intergroup National Clinical Trials Network Study S1001
- PMID: 32658627
- PMCID: PMC7479758
- DOI: 10.1200/JCO.20.00999
Positron Emission Tomography-Directed Therapy for Patients With Limited-Stage Diffuse Large B-Cell Lymphoma: Results of Intergroup National Clinical Trials Network Study S1001
Erratum in
-
Errata.J Clin Oncol. 2020 Oct 10;38(29):3459. doi: 10.1200/JCO.20.02475. J Clin Oncol. 2020. PMID: 33027606 Free PMC article. No abstract available.
Abstract
Purpose: Diffuse large B-cell lymphoma (DLBCL) presents as a limited-stage disease in 25% to 30% of patients, with better overall survival (OS) than that for advanced-stage disease but with continuous relapse regardless of treatment approach. The preferred treatment is abbreviated rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and radiation therapy. On the basis of promising results of positron emission tomography (PET)-directed treatment approaches, we designed a National Clinical Trials Network (NCTN) study to improve outcomes and decrease toxicity.
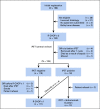
Methods: Patients with nonbulky (< 10 cm) stage I/II untreated DLBCL received 3 cycles of standard R-CHOP therapy and underwent a centrally reviewed interim PET/computed tomography scan (iPET). Those with a negative iPET proceeded with 1 additional cycle of R-CHOP, whereas those with a positive iPET received involved field radiation therapy followed by ibritumomab tiuxetan radioimmunotherapy.
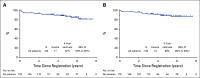
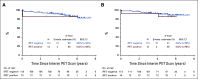
Results: Of 158 patients enrolled, 132 were eligible and 128 underwent iPET, which was positive in 14 (11%) of the patients. With a median follow-up of 4.92 years (range, 1.1-7.7 years), only 6 patients progressed and 3 died as a result of lymphoma. Eleven patients died as a result of nonlymphoma causes at a median age of 80 years. The 5-year progression-free survival estimate was 87% (95% CI, 79% to 92%) and the OS estimate was 89% (95% CI, 82% to 94%), with iPET-positive and iPET-negative patients having similar outcomes.
Conclusion: To our knowledge, S1001 is the largest prospective study in the United States of limited-stage DLBCL in the rituximab era, with the best NCTN results in this disease subset. With PET-directed therapy, 89% of the patients with a negative iPET received R-CHOP × 4, and only 11% had a positive iPET and required radiation, with both groups having excellent outcomes. The trial establishes R-CHOP × 4 alone as the new standard approach to limited-stage disease for the absolute majority of patients.
Figures
Comment in
-
Reply to E. Hawkes et al.J Clin Oncol. 2020 Dec 10;38(35):4222-4223. doi: 10.1200/JCO.20.02856. Epub 2020 Oct 26. J Clin Oncol. 2020. PMID: 33104437 No abstract available.
-
Caution in Expanding the Use of Abbreviated R-CHOP to Poor-Risk Limited-Stage DLBCL.J Clin Oncol. 2020 Dec 10;38(35):4221-4222. doi: 10.1200/JCO.20.02301. Epub 2020 Oct 26. J Clin Oncol. 2020. PMID: 33104442 No abstract available.
-
Interim positron emission tomography in limited-stage diffuse large B cell lymphoma: A modern will-o'-the-wisp?Natl Med J India. 2021 Jan-Feb;34(1):37-39. doi: 10.4103/0970-258X.323459. Natl Med J India. 2021. PMID: 34397004 No abstract available.
References
-
- Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21–26. - PubMed
-
- Miller TP. The limits of limited stage lymphoma. J Clin Oncol. 2004;22:2982–2984. - PubMed
-
- Shenkier TN, Voss N, Fairey R, et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: An 18-year experience from the British Columbia Cancer Agency. J Clin Oncol. 2002;20:197–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

