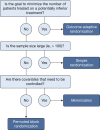
Randomized Controlled Trials
- PMID: 32658656
- PMCID: PMC8176647
- DOI: 10.1016/j.chest.2020.03.013
Randomized Controlled Trials
Abstract
Randomized controlled trials (RCTs) are considered the highest level of evidence to establish causal associations in clinical research. There are many RCT designs and features that can be selected to address a research hypothesis. Designs of RCTs have become increasingly diverse as new methods have been proposed to evaluate increasingly complex scientific hypotheses. This article reviews the principles and general concepts behind many common RCT designs and introduces newer designs that have been proposed, such as adaptive and cluster randomized trials. A focus on the many choices for randomization within an RCT is described, along with their potential tradeoffs. To illustrate their diversity, examples of RCTs from the literature are provided. Statistical considerations, such as power and type I error rates, are discussed with the intention of providing practical guidance about how to specify study hypotheses that address the scientific question while being statistically appropriate. Finally, the freely available Consolidated Standards of Reporting Trials guidelines and US Food and Drug Administration guidance documents are introduced, along with a set of guidelines one should consider when planning an RCT or reviewing RCTs submitted for publication in peer-reviewed academic journals.
Keywords: biostatistics; clinical trials; randomized controlled trials; study design.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sackett D.L. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(suppl 2):2s–4s. - PubMed
-
- US Department of Health and Human Services Food and Drug Administration. Guidance for Industry: Choice of Control Group and Related Issues in Clinical Trials. 2001. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed May 15, 2020.
-
- Hemming K., Haines T.P., Chilton P.J., Girling A.J., Lilford R.J. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources