Multiparameter Kinetic Analysis for Covalent Fragment Optimization by Using Quantitative Irreversible Tethering (qIT)
- PMID: 32659037
- PMCID: PMC7754465
- DOI: 10.1002/cbic.202000457
Multiparameter Kinetic Analysis for Covalent Fragment Optimization by Using Quantitative Irreversible Tethering (qIT)
Abstract
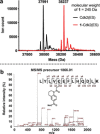
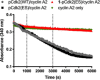
Chemical probes that covalently modify cysteine residues in a protein-specific manner are valuable tools for biological investigations. Covalent fragments are increasingly implemented as probe starting points, but the complex relationship between fragment structure and binding kinetics makes covalent fragment optimization uniquely challenging. We describe a new technique in covalent probe discovery that enables data-driven optimization of covalent fragment potency and selectivity. This platform extends beyond the existing repertoire of methods for identifying covalent fragment hits by facilitating rapid multiparameter kinetic analysis of covalent structure-activity relationships through the simultaneous determination of Ki , kinact and intrinsic reactivity. By applying this approach to develop novel probes against electrophile-sensitive kinases, we showcase the utility of the platform in hit identification and highlight how multiparameter kinetic analysis enabled a successful fragment-merging strategy.
Keywords: Cdk2; covalent fragments; covalent inhibition kinetics; electrophile-sensitive inhibition; fragment-based drug discovery.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Figures