Kinetic and Spectroscopic Characterization of the Catalytic Ternary Complex of Tryptophan 2,3-Dioxygenase
- PMID: 32659080
- PMCID: PMC7470927
- DOI: 10.1021/acs.biochem.0c00179
Kinetic and Spectroscopic Characterization of the Catalytic Ternary Complex of Tryptophan 2,3-Dioxygenase
Abstract
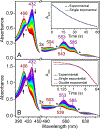
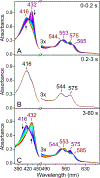
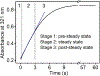
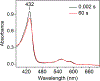
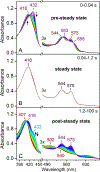
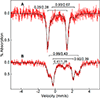
The first step of the kynurenine pathway for l-tryptophan (l-Trp) degradation is catalyzed by heme-dependent dioxygenases, tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase. In this work, we employed stopped-flow optical absorption spectroscopy to study the kinetic behavior of the Michaelis complex of Cupriavidus metallidurans TDO (cmTDO) to improve our understanding of oxygen activation and initial oxidation of l-Trp. On the basis of the stopped-flow results, rapid freeze-quench (RFQ) experiments were performed to capture and characterize this intermediate by Mössbauer spectroscopy. By incorporating the chlorite dismutase-chlorite system to produce high concentrations of solubilized O2, we were able to capture the Michaelis complex of cmTDO in a nearly quantitative yield. The RFQ-Mössbauer results confirmed the identity of the Michaelis complex as an O2-bound ferrous species. They revealed remarkable similarities between the electronic properties of the Michaelis complex and those of the O2 adduct of myoglobin. We also found that the decay of this reactive intermediate is the rate-limiting step of the catalytic reaction. An inverse α-secondary substrate kinetic isotope effect was observed with a kH/kD of 0.87 ± 0.03 when (indole-d5)-l-Trp was employed as the substrate. This work provides an important piece of spectroscopic evidence of the chemical identity of the Michaelis complex of bacterial TDO.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
Similar articles
-
Chemical rescue of the distal histidine mutants of tryptophan 2,3-dioxygenase.J Am Chem Soc. 2012 Jul 25;134(29):12209-18. doi: 10.1021/ja304164b. Epub 2012 Jul 13. J Am Chem Soc. 2012. PMID: 22742206
-
Initial O₂ Insertion Step of the Tryptophan Dioxygenase Reaction Proposed by a Heme-Modification Study.Biochemistry. 2015 Jun 16;54(23):3604-16. doi: 10.1021/acs.biochem.5b00048. Epub 2015 Jun 2. Biochemistry. 2015. PMID: 25996254
-
EPR and Mössbauer spectroscopy show inequivalent hemes in tryptophan dioxygenase.J Am Chem Soc. 2010 Jan 27;132(3):1098-109. doi: 10.1021/ja908851e. J Am Chem Soc. 2010. PMID: 20047315 Free PMC article.
-
Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase.Biochem Soc Trans. 2009 Apr;37(Pt 2):408-12. doi: 10.1042/BST0370408. Biochem Soc Trans. 2009. PMID: 19290871 Review.
-
Structure and reaction mechanism in the heme dioxygenases.Biochemistry. 2011 Apr 12;50(14):2717-24. doi: 10.1021/bi101732n. Epub 2011 Mar 18. Biochemistry. 2011. PMID: 21361337 Free PMC article. Review.
Cited by
-
Tryptophan Can Promote Oxygen Reduction to Water in a Biosynthetic Model of Heme Copper Oxidases.Biochemistry. 2023 Jan 17;62(2):388-395. doi: 10.1021/acs.biochem.2c00300. Epub 2022 Oct 10. Biochemistry. 2023. PMID: 36215733 Free PMC article.
-
Elucidating ligand interactions and small-molecule activation in the pyrrolnitrin biosynthetic enzyme PrnB.J Biol Chem. 2025 Feb;301(2):108123. doi: 10.1016/j.jbc.2024.108123. Epub 2024 Dec 25. J Biol Chem. 2025. PMID: 39725034 Free PMC article.
-
Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria.Nat Chem Biol. 2023 Nov;19(11):1415-1422. doi: 10.1038/s41589-023-01416-0. Epub 2023 Aug 31. Nat Chem Biol. 2023. PMID: 37653171
-
Secondary Sphere Lewis Acid Activated Heme Superoxo Adducts Mimic Crucial Non-Covalent Interactions in IDO/TDO Heme Dioxygenases.Chemistry. 2024 Dec 5;30(68):e202402310. doi: 10.1002/chem.202402310. Epub 2024 Nov 12. Chemistry. 2024. PMID: 39222484
-
Oxygen versus Sulfur Coordination in Cobalt Superoxo Complexes: Spectroscopic Properties, O2 Binding, and H-Atom Abstraction Reactivity.Inorg Chem. 2023 Jan 9;62(1):392-400. doi: 10.1021/acs.inorgchem.2c03484. Epub 2022 Dec 20. Inorg Chem. 2023. PMID: 36538786 Free PMC article.
References
-
- Sono M, Roach MP, Coulter ED, and Dawson JH (1996) Heme-containing oxygenases. Chem. Rev 96, 2841–2888. - PubMed
-
- Kal S, and Que L (2017) Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants. JBIC, J. Biol. Inorg. Chem 22, 339–365. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

