Infection of a Lepidopteran Cell Line with Deformed Wing Virus
- PMID: 32659903
- PMCID: PMC7412015
- DOI: 10.3390/v12070739
Infection of a Lepidopteran Cell Line with Deformed Wing Virus
Abstract
Many attempts to develop a reliable cell cultured-based system to study honey bee virus infections have encountered substantial difficulties. We investigated the ability of a cell line from a heterologous insect to sustain infection by a honey bee virus. For this purpose, we infected the Lepidopteran hemocytic cell line (P1) with Deformed wing virus (DWV). The genomic copies of DWV increased upon infection, as monitored by quantitative RT-PCR. Moreover, a tagged-primer-based RT-PCR analysis showed the presence of DWV negative-sense RNA in the cells, indicating virus replication. However, the DWV from infected cells was mildly infectious to P1 cells. Similar results were obtained when the virus was injected into Apis mellifera pupae. Thus, though the virus yields from the infected cells appeared to be very low, we show for the first time that DWV can replicate in a heterologous cell line. Given the availability of many other insect cell lines, our study paves the way for future exploration in this direction. In the absence of adequate A. mellifera cell lines, exploring the ability of alternative cell lines to enable honey bee virus infections could provide the means to study and understand the viral infectious cycle at the cellular level and facilitate obtaining purified isolates of these viruses.
Keywords: Deformed wing virus; Lepidopteran cells; honey bee virus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

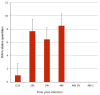

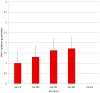
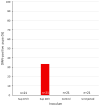
References
-
- Beaurepaire A., Doublet V., De Miranda J.R., Piot N., Antunez K., Campbell E., Chantawannakul P., Chejanovsky N., Gajda A., Heerman M., et al. Diversity and global distribution of viruses of the western honey bee, Apis mellifera. Insects. 2020;11:239. doi: 10.3390/insects11040239. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

