Development and Validation of a Deep Learning System for Diagnosing Glaucoma Using Optical Coherence Tomography
- PMID: 32659918
- PMCID: PMC7408821
- DOI: 10.3390/jcm9072167
Development and Validation of a Deep Learning System for Diagnosing Glaucoma Using Optical Coherence Tomography
Abstract
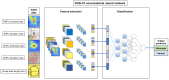
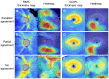
This study aimed to develop and validate a deep learning system for diagnosing glaucoma using optical coherence tomography (OCT). A training set of 1822 eyes (332 control, 1490 glaucoma) with 7288 OCT images, an internal validation set of 425 eyes (104 control, 321 glaucoma) with 1700 images, and an external validation set of 355 eyes (108 control, 247 glaucoma) with 1420 images were included. Deviation and thickness maps of retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GCIPL) analyses were used to develop the deep learning system for glaucoma diagnosis based on the visual geometry group deep convolutional neural network (VGG-19) model. The diagnostic abilities of deep learning models using different OCT maps were evaluated, and the best model was compared with the diagnostic results produced by two glaucoma specialists. The glaucoma-diagnostic ability was highest when the deep learning system used the RNFL thickness map alone (area under the receiver operating characteristic curve (AUROC) 0.987), followed by the RNFL deviation map (AUROC 0.974), the GCIPL thickness map (AUROC 0.966), and the GCIPL deviation map (AUROC 0.903). Among combination sets, use of the RNFL and GCIPL deviation map showed the highest diagnostic ability, showing similar results when tested via an external validation dataset. The inclusion of the axial length did not significantly affect the diagnostic performance of the deep learning system. The location of glaucomatous damage showed generally high level of agreement between the heatmap and the diagnosis of glaucoma specialists, with 90.0% agreement when using the RNFL thickness map and 88.0% when using the GCIPL thickness map. In conclusion, our deep learning system showed high glaucoma-diagnostic abilities using OCT thickness and deviation maps. It also showed detection patterns similar to those of glaucoma specialists, showing promising results for future clinical application as an interpretable computer-aided diagnosis.
Keywords: deep learning system; diagnostic ability; ganglion cell–inner plexiform layer; glaucoma; retinal nerve fiber layer; spectral-domain optical coherence tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McCarthy J., Minsky M.L., Rochester N., Shannon C.E. A proposal for the dartmouth summer research project on artificial intelligence, 31 August, 1955. AI Mag. 2006;27:12–14. doi: 10.1609/aimag.v27i4.1904. - DOI
-
- Ting D.S.W., Peng L., Varadarajan A.V., Keane P.A., Burlina P.M., Chiang M.F., Schmetterer L., Pasquale L.R., Bressler N.M., Webster D.R., et al. Deep learning in ophthalmology: The technical and clinical considerations. Prog. Retin. Eye Res. 2019;72:100759. doi: 10.1016/j.preteyeres.2019.04.003. - DOI - PubMed
-
- Leung C.K., Cheung C.Y., Weinreb R.N., Qiu Q., Liu S., Li H., Xu G., Fan N., Huang L., Pang C.P., et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: A variability and diagnostic performance study. Ophthalmology. 2009;116:1257–1263. doi: 10.1016/j.ophtha.2009.04.013. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

