Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study
- PMID: 32660025
- PMCID: PMC7400852
- DOI: 10.3390/nu12072041
Targeting the Intestinal Microbiota to Prevent Type 2 Diabetes and Enhance the Effect of Metformin on Glycaemia: A Randomised Controlled Pilot Study
Abstract
Early treatment may prevent or delay the onset of type 2 diabetes mellitus (T2DM) in individuals who are at high risk. Lifestyle interventions and the hypoglycemic drug metformin have been shown to reduce T2DM incidence. The effectiveness of such interventions may be enhanced by targeting environmental factors such as the intestinal microbiota, which has been proven to predict the response to lifestyle interventions and play a part in mediating the glucose-lowering effects of metformin. Shifts in the intestinal microbiota "towards a more balanced state" may promote glucose homeostasis by regulating short-chain fatty acids' production. This study aimed to investigate the safety and effect of a multi-strain probiotic on glycemic, inflammatory, and permeability markers in adults with prediabetes and early T2DM and to assess whether the probiotic can enhance metformin's effect on glycaemia. A randomised controlled pilot study was conducted in 60 adults with a BMI ≥ 25 kg/m2 and with prediabetes or T2DM (within the previous 12 months). The participants were randomised to a multi-strain probiotic (L. plantarum, L. bulgaricus, L. gasseri, B. breve, B. animalis sbsp. lactis, B. bifidum, S. thermophilus, and S. boulardii) or placebo for 12 weeks. Analyses of the primary outcome (fasting plasma glucose) and secondary outcomes, including, but not limited to, circulating lipopolysaccharide, zonulin, and short chain fatty acids and a metagenomic analysis of the fecal microbiome were performed at baseline and 12 weeks post-intervention. The results showed no significant differences in the primary and secondary outcome measures between the probiotic and placebo group. An analysis of a subgroup of participants taking metformin showed a decrease in fasting plasma glucose, HbA1c, insulin resistance, and zonulin; an increase in plasma butyrate concentrations; and an enrichment of microbial butyrate-producing pathways in the probiotic group but not in the placebo group. Probiotics may act as an adjunctive to metformin by increasing the production of butyrate, which may consequently enhance glucose management.
Keywords: intestinal microbiota; metformin; prediabetes; probiotics; short-chain fatty acids; type 2 diabetes mellitus.
Conflict of interest statement
The present study is part of a PhD project supported by The University of Sydney, Ecuadorian Government and Medlab Clinical Ltd. L.V. has received National Institute of Complementary Medicine and National Health and Medical Research Council of Australia grant funding and Industry support (FitBioceuticals Ltd., Swisse-Swisse Wellness and Parmalat) for research into probiotics. L.V. participates in research on probiotics at Medlab Clinical. The authors S.C., C.D.M., I.D.C., Y.Y.L., R.M., D.B., J.Z., C.H., J.K., T.I., M.S.G., E.S., G.T. and T.P. declare that there are no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
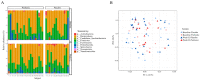

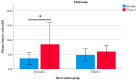
References
-
- Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

