Effect of Pregnancy on Unbound Raltegravir Concentrations in the ANRS 160 RalFe Trial
- PMID: 32661003
- PMCID: PMC7508578
- DOI: 10.1128/AAC.00759-20
Effect of Pregnancy on Unbound Raltegravir Concentrations in the ANRS 160 RalFe Trial
Abstract
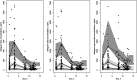
A population pharmacokinetic model was developed to explore the pharmacokinetics modification of unbound raltegravir during pregnancy. The RalFe ANRS160 study was a nonrandomized, open-label, multicenter trial enrolling HIV-infected pregnant women receiving a combined antiretroviral regimen containing 400 mg raltegravir twice daily. Biological samples were collected during the third trimester of pregnancy (between 30 and 37 weeks of gestational age) and at postpartum (4 to 6 weeks after delivery). A population pharmacokinetic model was developed with Monolix software. A total of 360 plasma samples were collected from 43 women during pregnancy and postpartum. The unbound raltegravir was described by a one-compartment model with a transit compartment with first-order absorption, evolving to bound raltegravir (by a linear binding to albumin) or metabolism to RAL-glucuronide or to a first-order elimination, with a circadian rhythm. During pregnancy, the absorption was decreased and delayed and the raltegravir elimination clearance and glucuronidation increased by 37%. Median total and unbound area under the curve from 0 to 12 h significantly decreased by 36% and 27% during pregnancy. Median total trough concentration (Ctrough) decreased significantly in the evening (28%); however, the median total Ctrough in the morning, unbound Ctrough in the morning, and unbound Ctrough in the evening showed a nonsignificant decrease of 16%, 1%, and 15%, respectively, during pregnancy compared to the postpartum period. This is the first study reporting the pharmacokinetics of unbound raltegravir during pregnancy. As unbound Ctrough did not significantly decrease during the third trimester, the pregnancy effect on raltegravir unbound concentrations was not considered clinically relevant. (This study has been registered at ClinicalTrials.gov under identifier NCT02099474.).
Keywords: HIV; population pharmacokinetics; pregnant women; raltegravir; unbound concentrations.
Copyright © 2020 American Society for Microbiology.
Figures


References
-
- WHO. 2020. Global guidance on criteria and processes for validation: elimination of Mother-to-Child Transmission of HIV and Syphilis. World Health Organization, Geneva, Switzerland.
-
- Cooper ER, Charurat M, Mofenson L, Hanson CI, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R, Blattner W, Group for the WITS. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 29:484. doi:10.1097/00126334-200204150-00009. - DOI - PubMed
-
- Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, McIntyre J, Gnanashanmugam D, Siberry GK, Coletti AS, Taha TE, Klingman KL, Martinson FE, Owor M, Violari A, Moodley D, Theron GB, Bhosale R, Bobat R, Chi BH, Strehlau R, Mlay P, Loftis AJ, Browning R, Fenton T, Purdue L, Basar M, Shapiro DE, Mofenson LM. 2016. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 375:1726–1737. doi:10.1056/NEJMoa1511691. - DOI - PMC - PubMed
-
- Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, Leroy V, Simonon A, Cartoux M, Combe P, Ouangré A, Ramon R, Ky-Zerbo O, Montcho C, Salamon R, Rouzioux C, Van de Perre P, Mandelbrot L. 1999. 6-Month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. Lancet 353:786–792. doi:10.1016/S0140-6736(98)11046-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

