Integrating molecular point-of-care testing for influenza into primary care: a mixed-methods feasibility study
- PMID: 32661013
- PMCID: PMC7363279
- DOI: 10.3399/bjgp20X710897
Integrating molecular point-of-care testing for influenza into primary care: a mixed-methods feasibility study
Abstract
Background: Molecular point-of-care testing (POCT) for influenza in primary care could influence clinical care and patient outcomes.
Aim: To assess the feasibility of incorporating influenza POCT into general practice in England.
Design and setting: A mixed-methods study conducted in six general practices that had not previously participated in respiratory virology sampling, which are part of the Royal College of General Practitioners Research and Surveillance Centre English sentinel surveillance network, from February 2019 to May 2019.
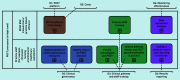
Method: A sociotechnical perspective was adopted using the Public Health England POCT implementation toolkit and business process modelling notation to inform qualitative analysis. Quantitative data were collected about the number of samples taken, their representativeness, and the virology results obtained, comparing them with the rest of the sentinel system over the same weeks.
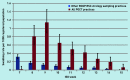
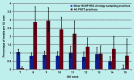
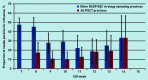
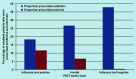
Results: A total of 312 POCTs were performed; 276 were used for quantitative analysis, of which 60 were positive for influenza and 216 were negative. The average swabbing rate was 0.4 per 1000 population and swab positivity was between 16.7% (n = 14/84) and 41.4% (n = 12/29). Given a positive influenza POCT result, the odds ratio of receiving an antiviral was 14.1 (95% confidence intervals [CI] = 2.9 to 70.0, P<0.001) and of receiving an antibiotic was 0.4 (95% CI = 0.2 to 0.8, P = 0.01), compared with patients with a negative result. Qualitative analysis showed that it was feasible for practices to implement POCT, but there is considerable variation in the processes used.
Conclusion: Testing for influenza using POCT is feasible in primary care and may improve antimicrobial use. However, further evidence from randomised trials of influenza POCT in general practice is needed.
Keywords: antibiotic; antiviral; general practice; influenza; medical record systems; point-of-care systems.
©The Authors.
Figures
References
-
- Dominguez A, Godoy P, Torner N. The effectiveness of influenza vaccination in different groups. Expert Rev Vaccines. 2016;15(6):751–764. - PubMed
-
- Public Health England . PHE guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza, Version 8.0. London: Public Health England; 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
