A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature
- PMID: 32661024
- PMCID: PMC7486666
- DOI: 10.1523/JNEUROSCI.3006-19.2020
A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature
Abstract
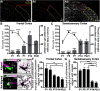
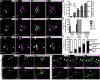
Microglia, a resident CNS macrophage, are dynamic cells, constantly extending and retracting their processes as they contact and functionally regulate neurons and other glial cells. There is far less known about microglia-vascular interactions, particularly under healthy steady-state conditions. Here, we use the male and female mouse cerebral cortex to show that a higher percentage of microglia associate with the vasculature during the first week of postnatal development compared with older ages and that the timing of these associations is dependent on the fractalkine receptor (CX3CR1). Similar developmental microglia-vascular associations were detected in the human brain. Using live imaging in mice, we found that juxtavascular microglia migrated when microglia are actively colonizing the cortex and became stationary by adulthood to occupy the same vascular space for nearly 2 months. Further, juxtavascular microglia at all ages associate with vascular areas void of astrocyte endfeet, and the developmental shift in microglial migratory behavior along vessels corresponded to when astrocyte endfeet more fully ensheath vessels. Together, our data provide a comprehensive assessment of microglia-vascular interactions. They support a mechanism by which microglia use the vasculature to migrate within the developing brain parenchyma. This migration becomes restricted on the arrival of astrocyte endfeet such that juxtavascular microglia become highly stationary and stable in the mature cortex.SIGNIFICANCE STATEMENT We report the first extensive analysis of juxtavascular microglia in the healthy, developing, and adult brain. Live imaging revealed that juxtavascular microglia within the cortex are highly motile and migrate along vessels as they are colonizing cortical regions. Using confocal, expansion, super-resolution, and electron microscopy, we determined that microglia associate with the vasculature at all ages in areas lacking full astrocyte endfoot coverage and motility of juxtavascular microglia ceases as astrocyte endfeet more fully ensheath the vasculature. Our data lay the fundamental groundwork to investigate microglia-astrocyte cross talk and juxtavascular microglial function in the healthy and diseased brain. They further provide a potential mechanism by which vascular interactions facilitate microglial colonization of the brain to later regulate neural circuit development.
Keywords: astrocytes; development; microglia; neural-immune; vasculature.
Copyright © 2020 the authors.
Figures





References
-
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K (2007) The fibrin-derived γ 377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 204:571–582. 10.1084/jem.20061931 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
