Generation and Characterization of Typhoid Toxin-Neutralizing Human Monoclonal Antibodies
- PMID: 32661121
- PMCID: PMC7504945
- DOI: 10.1128/IAI.00292-20
Generation and Characterization of Typhoid Toxin-Neutralizing Human Monoclonal Antibodies
Abstract
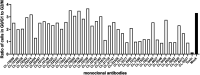
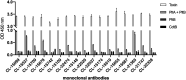
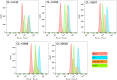
Typhoid toxin is a virulence factor of Salmonella enterica serovar Typhi, the causative agent of typhoid fever, and is thought to be responsible for the symptoms of severe disease. This toxin has a unique A2B5 architecture with two active subunits, the ADP ribosyl transferase PltA and the DNase CdtB, linked to a pentameric B subunit, which is alternatively made of PltB or PltC. Here, we describe the generation and characterization of typhoid toxin-neutralizing human monoclonal antibodies by immunizing genetically engineered mice that have a full set of human immunoglobulin variable region genes. We identified several monoclonal antibodies with strong in vitro and in vivo toxin-neutralizing activity and different mechanisms of toxin neutralization. These antibodies could serve as the basis for the development of novel therapeutic strategies against typhoid fever.
Keywords: Salmonella Paratyphi; Salmonella Typhi; bacterial pathogenesis; bacterial toxins; infectious diseases; monoclonal antibodies; therapeutics; typhoid fever.
Copyright © 2020 American Society for Microbiology.
Figures

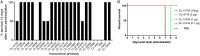

References
-
- Mogasale V, Maskery B, Ochiai R, Lee J, Mogasale V, Ramani E, Kim Y, Park J, Wierzba T. 2014. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2:e570–e580. doi:10.1016/S2214-109X(14)70301-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

