The molecular virology of coronaviruses
- PMID: 32661197
- PMCID: PMC7489918
- DOI: 10.1074/jbc.REV120.013930
The molecular virology of coronaviruses
Abstract
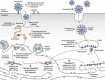
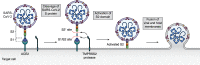
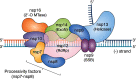
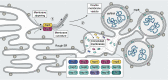
Few human pathogens have been the focus of as much concentrated worldwide attention as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19. Its emergence into the human population and ensuing pandemic came on the heels of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), two other highly pathogenic coronavirus spillovers, which collectively have reshaped our view of a virus family previously associated primarily with the common cold. It has placed intense pressure on the collective scientific community to develop therapeutics and vaccines, whose engineering relies on a detailed understanding of coronavirus biology. Here, we present the molecular virology of coronavirus infection, including its entry into cells, its remarkably sophisticated gene expression and replication mechanisms, its extensive remodeling of the intracellular environment, and its multifaceted immune evasion strategies. We highlight aspects of the viral life cycle that may be amenable to antiviral targeting as well as key features of its biology that await discovery.
Keywords: RNA polymerase; SARS-CoV-2; cellular immune response; coronavirus; endoplasmic reticulum; endoplasmic reticulum (ER); innate immunity; pathogenesis; plus-stranded RNA virus; viral replication; virology; virus; virus entry.
© 2020 Hartenian et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


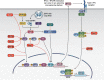
References
-
- He B., Zhang Y., Xu L., Yang W., Yang F., Feng Y., Xia L., Zhou J., Zhen W., Feng Y., Guo H., Zhang H., and Tu C. (2014) Identification of diverse alphacoronaviruses and genomic characterization of a novel severe acute respiratory syndrome-like coronavirus from bats in China. J. Virol. 88, 7070–7082 10.1128/JVI.00631-14 - DOI - PMC - PubMed
-
- Siu Y. L., Teoh K. T., Lo J., Chan C. M., Kien F., Escriou N., Tsao S. W., Nicholls J. M., Altmeyer R., Peiris J. S. M., Bruzzone R., and Nal B. (2008) The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 82, 11318–11330 10.1128/JVI.01052-08 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

