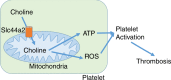
The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function
- PMID: 32661250
- PMCID: PMC7359028
- DOI: 10.1038/s41467-020-17254-w
The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function
Abstract
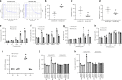
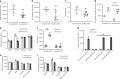
Genetic factors contribute to the risk of thrombotic diseases. Recent genome wide association studies have identified genetic loci including SLC44A2 which may regulate thrombosis. Here we show that Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial energetics. We find that Slc44a2 null mice (Slc44a2(KO)) have increased bleeding times and delayed thrombosis compared to wild-type (Slc44a2(WT)) controls. Platelets from Slc44a2(KO) mice have impaired activation in response to thrombin. We discover that Slc44a2 mediates choline transport into mitochondria, where choline metabolism leads to an increase in mitochondrial oxygen consumption and ATP production. Platelets lacking Slc44a2 contain less ATP at rest, release less ATP when activated, and have an activation defect that can be rescued by exogenous ADP. Taken together, our data suggest that mitochondria require choline for maximum function, demonstrate the importance of mitochondrial metabolism to platelet activation, and reveal a mechanism by which Slc44a2 influences thrombosis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Heit JA, et al. Familial segregation of venous thromboembolism. J. Thrombosis Haemost. 2004;2:731–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

