A six-attribute classification of genetic mosaicism
- PMID: 32661356
- PMCID: PMC8581815
- DOI: 10.1038/s41436-020-0877-3
A six-attribute classification of genetic mosaicism
Abstract
Mosaicism denotes an individual who has at least two populations of cells with distinct genotypes that are derived from a single fertilized egg. Genetic variation among the cell lines can involve whole chromosomes, structural or copy-number variants, small or single-nucleotide variants, or epigenetic variants. The mutational events that underlie mosaic variants occur during mitotic cell divisions after fertilization and zygote formation. The initiating mutational event can occur in any types of cell at any time in development, leading to enormous variation in the distribution and phenotypic effect of mosaicism. A number of classification proposals have been put forward to classify genetic mosaicism into categories based on the location, pattern, and mechanisms of the disease. We here propose a new classification of genetic mosaicism that considers the affected tissue, the pattern and distribution of the mosaicism, the pathogenicity of the variant, the direction of the change (benign to pathogenic vs. pathogenic to benign), and the postzygotic mutational mechanism. The accurate and comprehensive categorization and subtyping of mosaicisms is important and has potential clinical utility to define the natural history of these disorders, tailor follow-up frequency and interventions, estimate recurrence risks, and guide therapeutic decisions.
Keywords: mosaicism; mutational event; new classification; postzygotic.
Figures

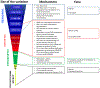

References
-
- De S Somatic Mosaicism in Healthy Human Tissues. Trends in genetics : TIG. 2011;27(6):217–223. - PubMed
-
- Vera-Rodriguez M, Rubio C. Assessing the True Incidence of Mosaicism in Preimplantation Embryos. Fertility and sterility. 2017;107(5):1107–1112. - PubMed
-
- Happle R Mosaicism in Human Skin. 1 ed: Springer-Verlag Berlin Heidelberg; 2014.
-
- Leija-Salazar M, Piette C, Proukakis C. Review: Somatic Mutations in Neurodegeneration. Neuropathology and applied neurobiology. 2018;44(3):267–285. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

