Myocardial infarction accelerates breast cancer via innate immune reprogramming
- PMID: 32661390
- PMCID: PMC7789095
- DOI: 10.1038/s41591-020-0964-7
Myocardial infarction accelerates breast cancer via innate immune reprogramming
Abstract
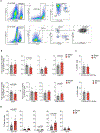
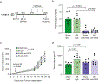
Disruption of systemic homeostasis by either chronic or acute stressors, such as obesity1 or surgery2, alters cancer pathogenesis. Patients with cancer, particularly those with breast cancer, can be at increased risk of cardiovascular disease due to treatment toxicity and changes in lifestyle behaviors3-5. While elevated risk and incidence of cardiovascular events in breast cancer is well established, whether such events impact cancer pathogenesis is not known. Here we show that myocardial infarction (MI) accelerates breast cancer outgrowth and cancer-specific mortality in mice and humans. In mouse models of breast cancer, MI epigenetically reprogrammed Ly6Chi monocytes in the bone marrow reservoir to an immunosuppressive phenotype that was maintained at the transcriptional level in monocytes in both the circulation and tumor. In parallel, MI increased circulating Ly6Chi monocyte levels and recruitment to tumors and depletion of these cells abrogated MI-induced tumor growth. Furthermore, patients with early-stage breast cancer who experienced cardiovascular events after cancer diagnosis had increased risk of recurrence and cancer-specific death. These preclinical and clinical results demonstrate that MI induces alterations in systemic homeostasis, triggering cross-disease communication that accelerates breast cancer.
Conflict of interest statement
Figures

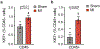


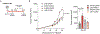






Comment in
-
Myocardial infarction promotes breast cancer recurrence.Nat Rev Clin Oncol. 2020 Sep;17(9):520. doi: 10.1038/s41571-020-0418-7. Nat Rev Clin Oncol. 2020. PMID: 32719418 No abstract available.
-
Cardiovascular disease and cancer communicate.Nat Rev Cancer. 2020 Oct;20(10):552. doi: 10.1038/s41568-020-0294-6. Nat Rev Cancer. 2020. PMID: 32753729 No abstract available.
-
MI drives progression of breast cancer by promoting a protumoural immune landscape.Nat Rev Cardiol. 2020 Oct;17(10):611. doi: 10.1038/s41569-020-0427-3. Nat Rev Cardiol. 2020. PMID: 32753736 No abstract available.
References
-
- Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS & Mackey JR Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 50, 1435–1441 (2007). - PubMed
-
- Hooning MJ, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 99, 365–375 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- P01 HL131481/HL/NHLBI NIH HHS/United States
- T32 HL098129/HL/NHLBI NIH HHS/United States
- P01 HL131478/HL/NHLBI NIH HHS/United States
- K23 HL125991/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R35 HL135799/HL/NHLBI NIH HHS/United States
- S10 OD021747/OD/NIH HHS/United States
- PJT159742/CIHR/Canada
- R01 CA234025/CA/NCI NIH HHS/United States
- R01 CA129059/CA/NCI NIH HHS/United States
- R01 DA049547/DA/NIDA NIH HHS/United States
- R01 HL132073/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

