Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits
- PMID: 32661395
- PMCID: PMC7483861
- DOI: 10.1038/s41593-020-0665-z
Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits
Abstract
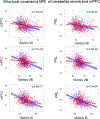
Cerebellar dysfunction has been demonstrated in autism spectrum disorders (ASDs); however, the circuits underlying cerebellar contributions to ASD-relevant behaviors remain unknown. In this study, we demonstrated functional connectivity between the cerebellum and the medial prefrontal cortex (mPFC) in mice; showed that the mPFC mediates cerebellum-regulated social and repetitive/inflexible behaviors; and showed disruptions in connectivity between these regions in multiple mouse models of ASD-linked genes and in individuals with ASD. We delineated a circuit from cerebellar cortical areas Right crus 1 (Rcrus1) and posterior vermis through the cerebellar nuclei and ventromedial thalamus and culminating in the mPFC. Modulation of this circuit induced social deficits and repetitive behaviors, whereas activation of Purkinje cells (PCs) in Rcrus1 and posterior vermis improved social preference impairments and repetitive/inflexible behaviors, respectively, in male PC-Tsc1 mutant mice. These data raise the possibility that these circuits might provide neuromodulatory targets for the treatment of ASD.
Conflict of interest statement
Ethics declarations
The authors declare no competing interests.
Figures
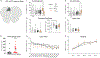










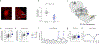
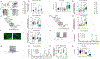

References
-
- Tsai PT Autism and cerebellar dysfunction: evidence from animal models. Semin. Fetal Neonatal Med 21, 349–355 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

