Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals
- PMID: 32661427
- PMCID: PMC10907227
- DOI: 10.1038/s41592-020-0889-6
Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals
Abstract
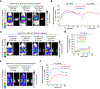
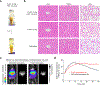
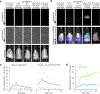
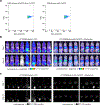
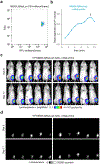
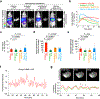
Sensitive detection of two biological events in vivo has long been a goal in bioluminescence imaging. Antares, a fusion of the luciferase NanoLuc to the orange fluorescent protein CyOFP, has emerged as a bright bioluminescent reporter with orthogonal substrate specificity to firefly luciferase (FLuc) and its derivatives such as AkaLuc. However, the brightness of Antares in mice is limited by the poor solubility and bioavailability of the NanoLuc substrate furimazine. Here, we report a new substrate, hydrofurimazine, whose enhanced aqueous solubility allows delivery of higher doses to mice. In the liver, Antares with hydrofurimazine exhibited similar brightness to AkaLuc with its substrate AkaLumine. Further chemical exploration generated a second substrate, fluorofurimazine, with even higher brightness in vivo. We used Antares with fluorofurimazine to track tumor size and AkaLuc with AkaLumine to visualize CAR-T cells within the same mice, demonstrating the ability to perform two-population imaging with these two luciferase systems.
Figures

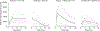



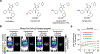


References
-
- Prescher JA & Contag CH. Guided by the light: visualizing biomolecular processes in living animals with bioluminescence. Current opinion in chemical biology 14, 80–89 (2010). - PubMed
-
- Contag CH et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochemistry and photobiology 66, 523–531 (1997). - PubMed
-
- Iwano S et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 359, 935–939 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

