Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts
- PMID: 32661868
- PMCID: PMC7456406
- DOI: 10.1007/s12015-020-10005-w
Nlrp3 Inflammasome Signaling Regulates the Homing and Engraftment of Hematopoietic Stem Cells (HSPCs) by Enhancing Incorporation of CXCR4 Receptor into Membrane Lipid Rafts
Abstract
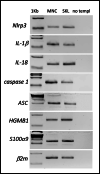
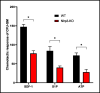
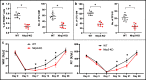
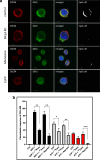
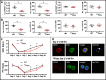
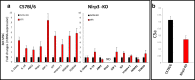
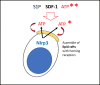
Fast and efficient homing and engraftment of hematopoietic stem progenitor cells (HSPCs) is crucial for positive clinical outcomes from transplantation. We found that this process depends on activation of the Nlrp3 inflammasome, both in the HSPCs to be transplanted and in the cells in the recipient bone marrow (BM) microenvironment. For the first time we provide evidence that functional deficiency in the Nlrp3 inflammasome in transplanted cells or in the host microenvironment leads to defective homing and engraftment. At the molecular level, functional deficiency of the Nlrp3 inflammasome in HSPCs leads to their defective migration in response to the major BM homing chemoattractant stromal-derived factor 1 (SDF-1) and to other supportive chemoattractants, including sphingosine-1-phosphate (S1P) and extracellular adenosine triphosphate (eATP). We report that activation of the Nlrp3 inflammasome increases autocrine release of eATP, which promotes incorporation of the CXCR4 receptor into membrane lipid rafts at the leading surface of migrating cells. On the other hand, a lack of Nlrp3 inflammasome expression in BM conditioned for transplantation leads to a decrease in expression of SDF-1 and danger-associated molecular pattern molecules (DAMPs), which are responsible for activation of the complement cascade (ComC), which in turn facilitates the homing and engraftment of HSPCs.
Keywords: Bone marrow sterile inflammation; Complement cascade; Extracellular nucleotides; Nlrp3 inflammasome; Purinergic signaling; Stem cell engraftment; Stem cell homing.
Conflict of interest statement
The authors have no financial interests to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

