The architecture and function of cardiac dyads
- PMID: 32661902
- PMCID: PMC7429583
- DOI: 10.1007/s12551-020-00729-x
The architecture and function of cardiac dyads
Abstract
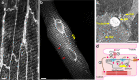
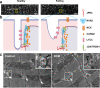
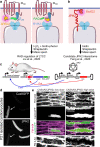
Cardiac excitation-contraction (EC) coupling, which links plasma membrane depolarization to activation of cardiomyocyte contraction, occurs at dyads, the nanoscopic microdomains formed by apposition of transverse (T)-tubules and junctional sarcoplasmic reticulum (jSR). In a dyadic junction, EC coupling occurs through Ca2+-induced Ca2+ release. Membrane depolarization opens voltage-gated L-type Ca2+ channels (LTCCs) in the T-tubule. The resulting influx of extracellular Ca2+ into the dyadic cleft opens Ca2+ release channels known as ryanodine receptors (RYRs) in the jSR, leading to the rapid increase in cytosolic Ca2+ that triggers sarcomere contraction. The efficacy of LTCC-RYR communication greatly affects a myriad of downstream intracellular signaling events, and it is controlled by many factors, including T-tubule and jSR structure, spatial distribution of ion channels, and regulatory proteins that closely regulate the activities of channels within dyads. Alterations in dyad architecture and/or channel activity are seen in many types of heart disease. This review will focus on the current knowledge regarding cardiac dyad structure and function, their alterations in heart failure, and new approaches to study the composition and function of dyads.
Keywords: Cardiac dyads; EC coupling; T-tubule; jSR.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Bers D (2001) Excitation-contraction coupling and cardiac contractile force. Springer Science & Business Media
-
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Bongianino R, Denegri M, Mazzanti A, et al. Allele-specific silencing of mutant mRNA rescues ultrastructural and arrhythmic phenotype in mice carriers of the R4496C mutation in the ryanodine receptor gene (RYR2) Circ Res. 2017;121:525–536. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

