Pharmacokinetic and Pharmacodynamic Studies of Elacestrant, A Novel Oral Selective Estrogen Receptor Degrader, in Healthy Post-Menopausal Women
- PMID: 32661909
- PMCID: PMC7511284
- DOI: 10.1007/s13318-020-00635-3
Pharmacokinetic and Pharmacodynamic Studies of Elacestrant, A Novel Oral Selective Estrogen Receptor Degrader, in Healthy Post-Menopausal Women
Erratum in
-
Correction to: Pharmacokinetic and Pharmacodynamic Studies of Elacestrant, A Novel Oral Selective Estrogen Receptor Degrader, in Healthy Post-Menopausal Women.Eur J Drug Metab Pharmacokinet. 2020 Oct;45(5):691-692. doi: 10.1007/s13318-020-00638-0. Eur J Drug Metab Pharmacokinet. 2020. PMID: 32862369 Free PMC article.
Abstract
Background and objectives: Advanced estrogen receptor-positive (ER+) breast cancer is currently treated with endocrine therapy. Elacestrant is a novel, nonsteroidal, selective estrogen receptor degrader with complex dose-related ER agonist/antagonist activity that is being developed as a treatment option for ER+ breast cancer.
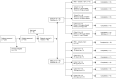
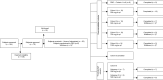
Methods: Two first-in-human phase 1 studies of elacestrant in healthy postmenopausal women (Study 001/Study 004) were conducted to determine its pharmacokinetic and pharmacodynamic profile as well as its safety and maximum tolerated dose.
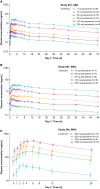
Results: In total, 140 postmenopausal subjects received at least one dose of study drug (114 received elacestrant and 26 received placebo). Single-ascending dose and multiple-ascending dose assessments showed that doses up to 1000 mg daily were safe and well tolerated, and the maximum tolerated dose was not reached. Oral administration of elacestrant had an absolute bioavailability of 10% and a mean half-life ranging from 27 to 47 h, reaching steady state after 5-6 days. Mean occupancy of the ER in the uterus after seven daily doses was 83% for 200 mg and 92% for 500 mg daily. The median ratio of elacestrant concentrations in the cerebral spinal fluid vs. plasma was 0.126% (500 mg dose) and 0.205% (200 mg dose). Most adverse events were related to the upper gastrointestinal tract.
Conclusions: These data demonstrate that elacestrant has good bioavailability when administered orally with a half-life that supports once-daily administration. Engagement of the ER and some ability to cross the blood-brain barrier was demonstrated in addition to an acceptable safety profile.
Conflict of interest statement
EFJDV: received payment from Radius Health, Inc., for the execution and analysis of the PET scans. AWJMG: no competing interest. YW, ST and MGC are employees and stockholders of Radius Health, Inc.
Figures

References
-
- Huang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol. 2005;58(6):611–616. doi: 10.1136/jcp.2004.022772. - DOI - PMC - PubMed
-
- National Comprehensive Cancer N. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer Version 2.2020-Feb 9, 2020.
-
- FASLODEX [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019.
-
- Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

